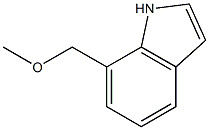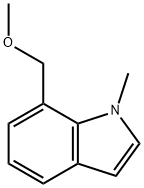
7-(METHOXYMETHYL)-1-METHYL-1H-INDOLE synthesis
- Product Name:7-(METHOXYMETHYL)-1-METHYL-1H-INDOLE
- CAS Number:1034895-78-7
- Molecular formula:C11H13NO
- Molecular Weight:175.23
1074-87-9
87 suppliers
$60.00/100mg

74-88-4
340 suppliers
$15.00/10g

1034895-78-7
7 suppliers
inquiry
Yield:1034895-78-7 89%
Reaction Conditions:
with sodium hydride in N,N-dimethyl-formamide at 0 - 20; for 1 h;
Steps:
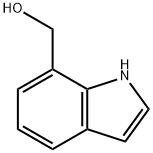
To a solution of (1 H-indol-7-yl)-methanol (0.50 g, 3.40 mmol) in dry DMF (6 ml_) cooled at 0 0C was added NaH (0.41 g, 10.19 mmol, 55% suspension in mineral oil), followed by methyl iodide (0.50 ml_, 8.15 mmol). The reaction mixture was allowed to warm to room temperature and stirred for 1 h. The reaction mixture was poured into ice-water and the solution was extracted with ethyl acetate. The ethyl acetate extract was dried over anhydrous Na2SO4, concentrated in vacuo and purified by column chromatography (ethylacetate:hexane; 1 :9) to afford the product (0.53 g, 89%) as slightly yellow oil.[000294] 1H NMR (CDCI3, 400 MHz) 3.36 (s, 3H), 4.04 (s, 3H), 4.77 (s, 2H), 6.46 (d, J=4.0 Hz, 1 H), 6.95-7.08 (m, 3H), 7.59 (dd, J=2.0, 8.0 Hz, 1 H), 13C NMR (CDCI3, 100 MHz) 35.4, 56.7, 72.7, 100.7, 118.4, 120.2, 121.5, 124.7, 129.9, 130.2, 134.6.
References:
WO2008/77138,2008,A1 Location in patent:Page/Page column 94