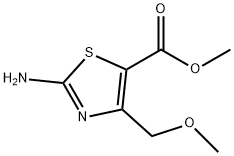
5-Thiazolecarboxylicacid,2-amino-4-(methoxymethyl)-,methylester(9CI) synthesis
- Product Name:5-Thiazolecarboxylicacid,2-amino-4-(methoxymethyl)-,methylester(9CI)
- CAS Number:733754-08-0
- Molecular formula:C7H10N2O3S
- Molecular Weight:202.23
Yield:733754-08-0 68%
Reaction Conditions:
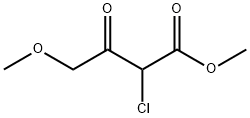
Stage #1: 4-methoxyacetoacetic acid methylesterwith sulfuryl dichloride in dichloromethane at 20; for 1 h;
Stage #2: thiourea;methyl 2-chloro-4-methoxy-3-oxobutanoate in ethanol; for 12 h;
Steps:
17
To a 100 mL round-bottomed flask was added methyl 4-methoxy-3-oxobutanoate (4.43 mL, 34.2 mmol) and DCM (30.00 mL). Sulfuryl chloride (2.91 mL, 35.9 mmol) was added dropwise with a syringe to the reaction mixture. The mixture was then stirred for 1 hour at 20° C. The solution was reduced to an oil under reduced pressure and dissolved in EtOH (50 mL). To this solution was added methyl 2-chloro-4-methoxy-3-oxobutanoate (6.18 g, 34 mmol) and thiourea (1.9 mL, 34 mmol). The reaction was then stirred at reflux for approximately 12 hours. LCMS indicated that the reaction was complete, and the solvent was removed under reduced pressure. Saturated aqueous sodium bicarbonate was added, and the resulting solid was filtered and recrystallized from water and <5 mL EtOH to give methyl 2-amino-4-(methoxymethyl)thiazole-5-carboxylate as rust colored crystals (4.85 g, 68%). LCMS (M+H) 203 calc. for C7H11N2O3S 203.2. 1H NMR (400 MHz, CD3OD): δ ppm 4.64 (s, 2H), 3.77 (s, 3H), 3.37 (s, 3H).
References:
US2007/173506,2007,A1 Location in patent:Page/Page column 37
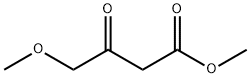
41051-15-4
300 suppliers
$6.00/5g

733754-08-0
14 suppliers
inquiry