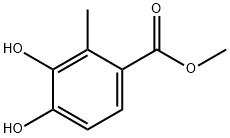
Benzoic acid, 3,4-dihydroxy-2-Methyl-, Methyl ester synthesis
- Product Name:Benzoic acid, 3,4-dihydroxy-2-Methyl-, Methyl ester
- CAS Number:740799-82-0
- Molecular formula:C9H10O4
- Molecular Weight:182.17

67-56-1
775 suppliers
$9.00/25ml
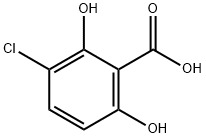
26754-77-8
21 suppliers
$65.00/100mg

740799-82-0
102 suppliers
$10.00/250mg
Yield:-
Reaction Conditions:
Stage #1: 2-methyl-3,4-dimethoxybenzoic acidwith boron tribromide in dichloromethane at 20; for 2 h;Cooling with ice;
Stage #2: methanol in dichloromethane at 20; for 48 h;Cooling with ice;
Steps:
103.1 Step (1): compound ii-38a → compound ii-38b
Boron tribromide (12.05 mL, 127 mmol) was added to a solution of compound ii-38a (10.0 g, 51.0 mmol, synthesized with reference to a method described in WO 2009/55077 A1) in dichloromathane (60 mL) while cooled with ice. The resultant solution was stirred at room temperature for 2 hours. The reaction liquid was added to methanol (100 mL) while cooled with ice. The resultant was stirred at room temperature for 2 days. The reaction liquid was diluted with dichloromethane/methanol, washed with a saturated salt solution, and dried over magnesium sulfate. Magnesium sulfate was filtrated off, and then the liquid was concentrated under reduced pressure. Thereto was added diisopropyl ether to precipitate a solid. The solid was collected by filtration, so as to yield compound ii-38b (2.86 g, 31%. The solid precipitated while the filtrate was separated to two phases was filtrated, and washed with water to yield compound ii-38b (3.30 g, 36%).1H-NMR (DMSO-d6) δ: 10.05 (1H, br s), 8.46 (1H, br s), 7.23 (1H, d, J = 8.46 Hz), 6.67 (1H, d, J = 8.46 Hz), 3.73 (3H, s), 2.34 (3H, s).
References:
EP2557082,2013,A1 Location in patent:Paragraph 0407; 0408

35490-07-4
18 suppliers
$450.00/1g

740799-82-0
102 suppliers
$10.00/250mg
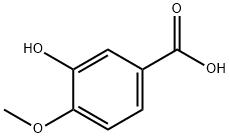
645-08-9
345 suppliers
$10.00/1g

740799-82-0
102 suppliers
$10.00/250mg
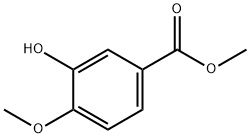
6702-50-7
200 suppliers
$11.00/1g

740799-82-0
102 suppliers
$10.00/250mg