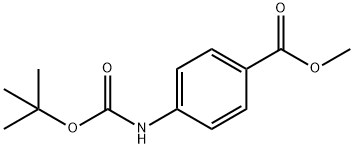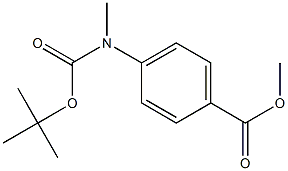
METHYL 4-(TERT-BUTOXYCARBONYL(METHYL)AMINO)BENZOATE synthesis
- Product Name:METHYL 4-(TERT-BUTOXYCARBONYL(METHYL)AMINO)BENZOATE
- CAS Number:741275-29-6
- Molecular formula:C14H19NO4
- Molecular Weight:265.305

24424-99-5
827 suppliers
$13.50/25G
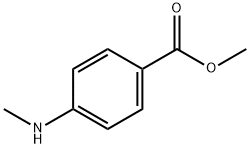
18358-63-9
109 suppliers
$15.00/1g

741275-29-6
9 suppliers
inquiry
Yield:741275-29-6 44%
Reaction Conditions:
with dmap in tetrahydrofuran at 20;
Steps:
Intermediate 283 methyl 4-[(tert-butoxycarbonyl)(methyl)amino]benzoate
To methyl 4-(methylamino)benzoate (3.99 g, 24.1 mmol) in tetrahydrofuran (48 ml, 590 mmol) was added di-tert-butyl dicarbonate (5.8 ml, 25 mmol) and N,N-dimethylpyridin-4-amine (295 mg, 2.41 mmol) and the reaction stirred overnight at room temperature. The reaction mixture was then diluted with ethylacetate, washed twice with water, once with a satured aqueous solution of sodium chloride, the organic phase then dried with sodium sulfate and concentrated in vacuo. The crude product was purified by flash-chromatography on silica gel (gradient of ethylacetate in cyclohexane, column: Biotage SNAP Ultra 50 g) to yield 2.79 g (100% purity, 44% yield) of the desired product. LC-MS (Method 9): Rt = 1.05 min; MS (ESIpos): m/z = 266 [M+H]+1H-NMR (400 MHz, dimethylsulfoxide-d6) δ [ppm]: 1.420 (16.00), 3.310 (2.45), 3.840 (5.81), 7.440 (1.41), 7.462 (1.53), 7.905 (1.63), 7.909 (0.53), 7.922 (0.51), 7.927 (1.44).
References:
WO2018/69222,2018,A1 Location in patent:Page/Page column 305

67-56-1
738 suppliers
$9.00/25ml
201230-82-2
1 suppliers
inquiry
639520-70-0
37 suppliers
$14.00/250mg

741275-29-6
9 suppliers
inquiry

24424-99-5
827 suppliers
$13.50/25G

619-45-4
386 suppliers
$6.00/1g

74-88-4
343 suppliers
$15.00/10g

741275-29-6
9 suppliers
inquiry