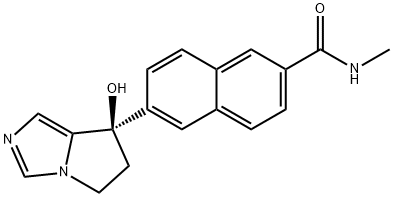
6-[7(R)-Hydroxy-6,7-dihydro-5H-pyrrolo[1,2-c]imidazol-7-yl]-N-methyl-2-naphthamide synthesis
- Product Name:6-[7(R)-Hydroxy-6,7-dihydro-5H-pyrrolo[1,2-c]imidazol-7-yl]-N-methyl-2-naphthamide
- CAS Number:752243-39-3
- Molecular formula:C18H17N3O2
- Molecular Weight:307.35
![6-(7-hydroxy-6,7-dihydro-5H-pyrrolo[1,2-c]iMidazol-7-yl)-N-Methyl-2-naphthaMide](/CAS/GIF/426219-32-1.gif)
426219-32-1
3 suppliers
inquiry
![6-[(7S)-7-Hydroxy-6,7-dihydro-5H-pyrrolo[1,2-c]imidazol-7-yl]-N-methyl-2-naphthalenecarboxamide](/CAS/GIF/566939-85-3.gif)
566939-85-3
81 suppliers
$81.00/5mg
![6-[7(R)-Hydroxy-6,7-dihydro-5H-pyrrolo[1,2-c]imidazol-7-yl]-N-methyl-2-naphthamide](/CAS/GIF/752243-39-3.gif)
752243-39-3
16 suppliers
inquiry
Yield:566939-85-3 49% ,752243-39-3 50%
Reaction Conditions:
with Chiralpak AD column in ethanol;hexane;Resolution of racemate;HPLC conditions;optical yield given as %ee;
Steps:
4.29. Optical resolution of 3c using preparative HPLC
Compound 3c (12.8 g, 41.6 mmol) was subjected to optical resolution by preparative HPLC using a Chiralpak AD column (50 mm × 500 mm) using hexane/ethanol (1/1) as an eluent, detected at 254 nm, to afford (-)-3c (6.40 g, 20.8 mmol, 50%) as a first elution and (+)-3c (6.32 g, 20.6 mmol, 49%) as a second elution. The spectral data were identical to those of the racemic 3c except for optical rotation.CommentCompound (-)-3c: -82.7 (c 0.526, MeOH); tR = 9.9 min (Chiralpak AD 4.6 mmID × 250mmL, hexane/ethanol = 1/1, 0.5 mL/min, at 254 nm); 99.8% ee. Compound (+)-3c: +83.8 (c 0.474, MeOH); tR = 19.1 min (Chiralpak AD 4.6 mmID × 250 mmL, hexane/ethanol = 1/1, 0.5 mL/min, at 254 nm); 99.8% ee.
References:
Ruchelman, Alexander L.;Man, Hon-Wah;Chen, Roger;Liu, Wei;Lu, Ling;Cedzik, Dorota;Zhang, Ling;Leisten, Jim;Collette, Alice;Narla, Rama Krishna;Raymon, Heather K.;Muller, George W. [Bioorganic and Medicinal Chemistry,2011,vol. 19,# 21,p. 6356 - 6374] Location in patent:experimental part
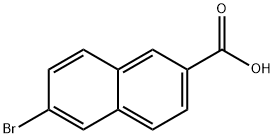
5773-80-8
304 suppliers
$10.00/1g
![6-[(7S)-7-Hydroxy-6,7-dihydro-5H-pyrrolo[1,2-c]imidazol-7-yl]-N-methyl-2-naphthalenecarboxamide](/CAS/GIF/566939-85-3.gif)
566939-85-3
81 suppliers
$81.00/5mg
![6-[7(R)-Hydroxy-6,7-dihydro-5H-pyrrolo[1,2-c]imidazol-7-yl]-N-methyl-2-naphthamide](/CAS/GIF/752243-39-3.gif)
752243-39-3
16 suppliers
inquiry
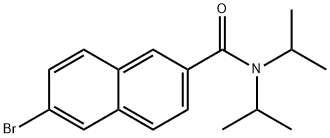
426219-46-7
3 suppliers
inquiry
![6-[(7S)-7-Hydroxy-6,7-dihydro-5H-pyrrolo[1,2-c]imidazol-7-yl]-N-methyl-2-naphthalenecarboxamide](/CAS/GIF/566939-85-3.gif)
566939-85-3
81 suppliers
$81.00/5mg
![6-[7(R)-Hydroxy-6,7-dihydro-5H-pyrrolo[1,2-c]imidazol-7-yl]-N-methyl-2-naphthamide](/CAS/GIF/752243-39-3.gif)
752243-39-3
16 suppliers
inquiry
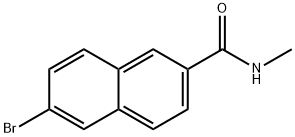
426219-35-4
77 suppliers
$90.00/100mg
![6-[(7S)-7-Hydroxy-6,7-dihydro-5H-pyrrolo[1,2-c]imidazol-7-yl]-N-methyl-2-naphthalenecarboxamide](/CAS/GIF/566939-85-3.gif)
566939-85-3
81 suppliers
$81.00/5mg
![6-[7(R)-Hydroxy-6,7-dihydro-5H-pyrrolo[1,2-c]imidazol-7-yl]-N-methyl-2-naphthamide](/CAS/GIF/752243-39-3.gif)
752243-39-3
16 suppliers
inquiry
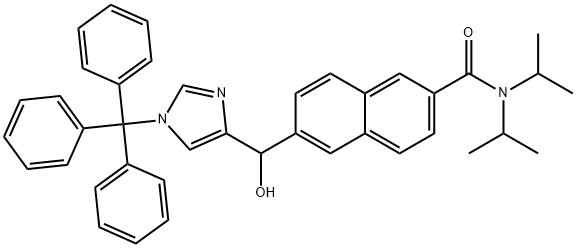
1346155-54-1
1 suppliers
inquiry
![6-[(7S)-7-Hydroxy-6,7-dihydro-5H-pyrrolo[1,2-c]imidazol-7-yl]-N-methyl-2-naphthalenecarboxamide](/CAS/GIF/566939-85-3.gif)
566939-85-3
81 suppliers
$81.00/5mg
![6-[7(R)-Hydroxy-6,7-dihydro-5H-pyrrolo[1,2-c]imidazol-7-yl]-N-methyl-2-naphthamide](/CAS/GIF/752243-39-3.gif)
752243-39-3
16 suppliers
inquiry