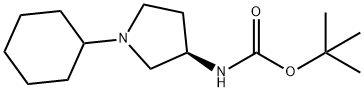
(R)-tert-Butyl 1-cyclohexylpyrrolidin-3-ylcarbamate synthesis
- Product Name:(R)-tert-Butyl 1-cyclohexylpyrrolidin-3-ylcarbamate
- CAS Number:762285-78-9
- Molecular formula:C15H28N2O2
- Molecular Weight:268.39

108-94-1
527 suppliers
$12.00/50g
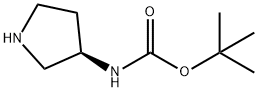
122536-77-0
311 suppliers
$9.00/1g

762285-78-9
7 suppliers
$309.00/1g
Yield:762285-78-9 96%
Reaction Conditions:
Stage #1: cyclohexanone;3-N-tert-butoxycarbonyl-3-(R)-aminopyrrolidine in methanol; for 0.333333 h;
Stage #2: with sodium cyanoborohydride in methanol; for 20 h;
Steps:
11A Step11 A :
(3R)- (+)-3- (tert-Butoxycarbonylamino) pyrrolidine (4.95 g, 26.6 mmol) and cyclohexanone (2.80 mL, 27.0 mmol) were dissolved in methanol (50 mL), and the mixture was stirred for 20 minutes. Sodium cyanoborohydride (2.04 g, 32.5 mmol) was added. After stirring for 20 hours, the mixture was quenched with aqueous sodium bicarbonate (40 mL), the methanol was removed UNDER vacuum, and the resulting residue was extracted with dichloromethane. The combined extracts were dried (MGS04) and concentrated to afford 6.82 g (96 % yield) of LLA as a colorless oil. LC-MS 269 (MH+)
References:
WO2004/81005,2004,A1 Location in patent:Page 53

108-94-1
527 suppliers
$12.00/50g
131878-23-4
98 suppliers
$8.00/1g

762285-78-9
7 suppliers
$309.00/1g