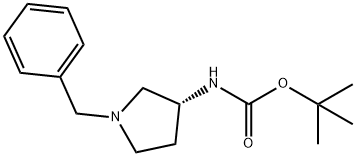
(3R)-(+)-1-BENZYL-3-(TERT-BUTOXYCARBONYLAMINO)PYRROLIDINE synthesis
- Product Name:(3R)-(+)-1-BENZYL-3-(TERT-BUTOXYCARBONYLAMINO)PYRROLIDINE
- CAS Number:131878-23-4
- Molecular formula:C16H24N2O2
- Molecular Weight:276.37

24424-99-5
821 suppliers
$13.50/25G
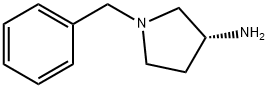
114715-39-8
233 suppliers
$18.00/250mg

131878-23-4
100 suppliers
$8.00/1g
Yield: 65.2%
Reaction Conditions:
Stage #1:(R)-1-benzyl-3-aminopyrrolidine with sodium hydrogencarbonate in water;acetonitrile at 20; for 0.166667 h;
Stage #2:di-tert-butyl dicarbonate in water;acetonitrile at 20;
Steps:
1 6.2.1 Synthesis of tert-butyl (R)-(1-benzylpyrrolidin-3-yl)carbamate, 2aa
Sodium bicarbonate (5.92 g, 70.5 mmol) in 118 mL of deionized water was added to (3R)-(+)-benzylaminopyrrolidine 1a (5.00 g, 28.4 mmol) solution in 118 mL of acetonitrile and the mixture was stirred at room temperature for 10 min. Di-tert-butyl dicarbonate (6.22 g, 28.5 mmol) was then added and the mixture was stirred at room temperature overnight. After the reaction, the solution was concentrated under reduced pressure and the residue was extracted with dichloromethane three times. The combined organic layers were dried over anhydrous sodium sulfate, filtered and concentrated. The residue was purified with flash column chromatography (methanol: dichloromethane = 2:98). Removing the solvent in vacuo provided 4.24 g of tert-butyl (R)-(1-benzylpyrrolidin-3-yl)carbamate (65.2% yield). 1H NMR (400 MHz, CDCl3) δ 7.36-7.26 (m, 5H), 4.86 (bs, 1H), 4.18 (bs, 1H), 3.61 (s, 2H), 2.79 (bs, 1H), 2.65-2.61 (m, 1H), 2.54 (d, J = 8.0 Hz, 1H), 2.34-2.25 (m, 2H), 1.61-1.51 (m, 1H), 1.46 (s, 9H). [α]D +2.5° (c 0.620, CHCl3).
References:
Chough, Chieyeon;Joung, Misuk;Lee, Sunmin;Lee, Jaemin;Kim, Jong Hoon;Kim, B. Moon [Bioorganic and Medicinal Chemistry,2018,vol. 26,# 8,p. 1495 - 1510]

100-52-7
945 suppliers
$17.30/2g
122536-77-0
312 suppliers
$9.00/1g

131878-23-4
100 suppliers
$8.00/1g
101385-90-4
297 suppliers
$12.00/1g

131878-23-4
100 suppliers
$8.00/1g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

131878-23-4
100 suppliers
$8.00/1g
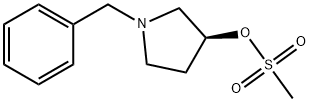
118354-71-5
20 suppliers
inquiry

131878-23-4
100 suppliers
$8.00/1g