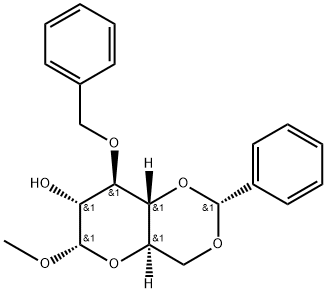
α-D-Glucopyranoside, methyl 3-O-(phenylmethyl)-4,6-O-[(R)-phenylmethylene]- synthesis
- Product Name:α-D-Glucopyranoside, methyl 3-O-(phenylmethyl)-4,6-O-[(R)-phenylmethylene]-
- CAS Number:76419-48-2
- Molecular formula:C21H24O6
- Molecular Weight:372.42
Yield:76419-48-2 94%
Reaction Conditions:
Stage #1: methyl 2,3,4,6-tetrakis-O-trimethylsilyl-α-D-glucopyranoside;benzaldehyde in dichloromethane at -78; for 0.5 h;Inert atmosphere;
Stage #2: with trimethylsilyl trifluoromethanesulfonate in dichloromethane; for 1 h;Inert atmosphere;
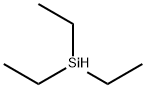
Stage #3: with triethylsilane in dichloromethane; for 3 h;Inert atmosphere;
Steps:
Methyl 3-O-benzyl-(R)-4,6-O-benzylidene-α-D-glucopyranoside (15) (Wang et al., 2008).
Compound 14 (1.73 g, 3.58 mmol) and PhCHO (380 μL, 3.7 mmol) was dissolved in dry DCM (30mL) and stirred at -78 °C under nitrogen for 30 min. Then TMSOTf (97 μL, 0.54 mmol) in DCM (1mL) was added drop wise and the mixture was allowed to stir for 1 h. Then PhCHO (440 μL, 4.30mmol) and Et3SiH (630 μL, 3.9 mmol) in DCM (2 mL) was added drop wise and the mixture wasstirred for another 3 h. The mixture was then heated to rt, diluted with EtOAc and washed twicewith sat. NaHCO3 (aq), and brine and finally dried over MgSO4 and evaporated under reducedpressure giving 1.4 g of crude product. The crude product was separated by flash columnchromatography with solvent system Hex:EtOAc 3:1 giving pure compound 15 (1.25 g, 94%). TLC(Hex/EtOAc 9:1): Rf 0.36; 1H-NMR (500 MHz, CDCl3): 7.45-7.48 (m, 2 H, ArH), 7.23-7.38 (m, 8H, ArH), 5.55 (s, 1 H, PhCH), 4.75-4.78 (m, 2 H, H-1, PhCH2), 4.27 (dd, J = 4.6 Hz and 10.0 Hz, 1H, H-4), 3.79-3.84 (m, 2 H, H-3, H-6), 3.69-3.76 (m, 2 H, H-2, H-5), 3.62 (at, J = 9.3 Hz, 1 H, H-6’), 3.43 (s, 3 H, OMe), 2.30 (d, J = 7.4 Hz, OH). 13C-NMR (125 MHz, CDCl3): 138.6, 137.5,129.1, 128.5, 128.4, 128.1, 127.8, 126.2, 101.4, 100.0, 82.1, 79.0, 74.9, 72.6, 69.2, 62.7, 55.5
References:
Norberg, Oscar;Wu, Bin;Thota, Niranjan;Ge, Jian-Tao;Fauquet, Germain;Saur, Ann-Kathrin;Aastrup, Teodor;Dong, Hai;Yan, Mingdi;Ramstr?m, Olof [Carbohydrate Research,2017,vol. 452,p. 35 - 42] Location in patent:supporting information
617-86-7
502 suppliers
$9.00/5g
![α-D-Glucopyranoside, methyl 4,6-O-[(R)-phenylmethylene]-2,3-O-[(S)-phenylmethylene]-](/CAS/20210305/GIF/148406-80-8.gif)
148406-80-8
0 suppliers
inquiry
![α-D-Glucopyranoside, methyl 3-O-(phenylmethyl)-4,6-O-[(R)-phenylmethylene]-2-O-(triethylsilyl)-](/CAS/20210305/GIF/1004556-10-8.gif)
1004556-10-8
0 suppliers
inquiry
![α-D-Glucopyranoside, methyl 3-O-(phenylmethyl)-4,6-O-[(R)-phenylmethylene]-](/CAS/20211123/GIF/76419-48-2.gif)
76419-48-2
1 suppliers
inquiry
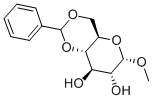
3162-96-7
189 suppliers
$11.19/1G

100-39-0
425 suppliers
$10.00/10g

3162-96-7
189 suppliers
$11.19/1G
![α-D-Glucopyranoside, methyl 2-O-(phenylmethyl)-4,6-O-[(R)-phenylmethylene]-](/CAS/20211123/GIF/76375-66-1.gif)
76375-66-1
1 suppliers
inquiry
![α-D-Glucopyranoside, methyl 3-O-(phenylmethyl)-4,6-O-[(R)-phenylmethylene]-](/CAS/20211123/GIF/76419-48-2.gif)
76419-48-2
1 suppliers
inquiry
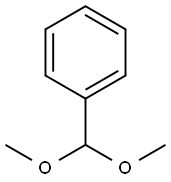
1125-88-8
347 suppliers
$6.00/25g
![α-D-Glucopyranoside, methyl 3-O-(phenylmethyl)-4,6-O-[(R)-phenylmethylene]-](/CAS/20211123/GIF/76419-48-2.gif)
76419-48-2
1 suppliers
inquiry


![α-D-Glucopyranoside, methyl 4,6-O-[(R)-phenylmethylene]-2,3-bis-O-(trimethylsilyl)-](/CAS/20210305/GIF/473711-72-7.gif)