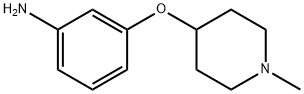
Benzenamine, 3-[(1-methyl-4-piperidinyl)oxy]- (9CI) synthesis
- Product Name:Benzenamine, 3-[(1-methyl-4-piperidinyl)oxy]- (9CI)
- CAS Number:790667-66-2
- Molecular formula:C12H18N2O
- Molecular Weight:206.28
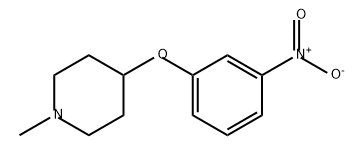
872512-95-3
0 suppliers
inquiry
![Benzenamine, 3-[(1-methyl-4-piperidinyl)oxy]- (9CI)](/CAS/GIF/790667-66-2.gif)
790667-66-2
10 suppliers
inquiry
Yield:790667-66-2 97%
Reaction Conditions:
with Pd/C;hydrogen in tetrahydrofuran at 20; under 775.743 Torr; for 4 h;
Steps:
3 Step 3
To a solution of 1 -methyl-4-(3-nitrophenoxy)piperidine (320 mg, 1 .35 mmol, 1 .00 eq) in tetrahydrofuran (5.00 mL) was added Pd/C 10% weight on C ( 100 mg, 1 .00 eq). The reaction was stirred under hydrogen atmosphere ( 1 5 psi) at 20°C for 4 h. The mixture was filtered, and the filtrate was concentrated under reduced pressure to afford 3-[(1 -methyl-4- piperidyl)oxy]aniline (270 mg, 1 .31 mmol, 97% yield) as a yellow solid.
References:
WO2021/69705,2021,A1 Location in patent:Page/Page column 228-229
![Acetamide, N-[3-[(1-methyl-4-piperidinyl)oxy]phenyl]-](/CAS/20210305/GIF/802541-79-3.gif)
802541-79-3
0 suppliers
inquiry
![Benzenamine, 3-[(1-methyl-4-piperidinyl)oxy]- (9CI)](/CAS/GIF/790667-66-2.gif)
790667-66-2
10 suppliers
inquiry
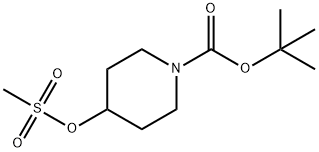
141699-59-4
233 suppliers
$6.00/1g
![Benzenamine, 3-[(1-methyl-4-piperidinyl)oxy]- (9CI)](/CAS/GIF/790667-66-2.gif)
790667-66-2
10 suppliers
inquiry
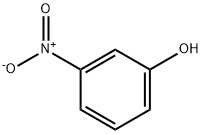
554-84-7
8 suppliers
$12.00/5g
![Benzenamine, 3-[(1-methyl-4-piperidinyl)oxy]- (9CI)](/CAS/GIF/790667-66-2.gif)
790667-66-2
10 suppliers
inquiry
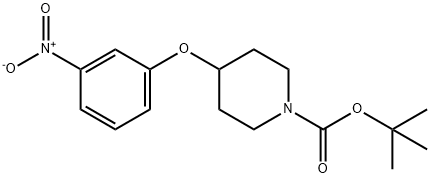
586412-88-6
12 suppliers
inquiry
![Benzenamine, 3-[(1-methyl-4-piperidinyl)oxy]- (9CI)](/CAS/GIF/790667-66-2.gif)
790667-66-2
10 suppliers
inquiry