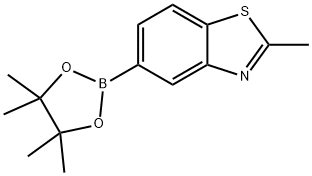
Benzothiazole, 2-methyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)- synthesis
- Product Name:Benzothiazole, 2-methyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-
- CAS Number:791614-90-9
- Molecular formula:C14H18BNO2S
- Molecular Weight:275.17
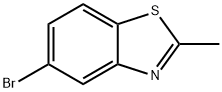
63837-11-6
157 suppliers
$15.00/1g

73183-34-3
564 suppliers
$6.00/5g

791614-90-9
17 suppliers
inquiry
Yield:791614-90-9 95%
Reaction Conditions:
with palladium (II) [1,1'-bis(diphenylphosphanyl)ferrocene] dichloride;anhydrous potassium acetate in N,N-dimethyl-formamide at 80; for 24 h;
Steps:
1 Preparation of compound a-1 (5-BOONS)
5-bromo-2-methylbenzo[d]thiazole (5-bromo-2-methylbenzo[d]thiazole, 20 g, 87.7 mmol), 4,4,4',4',5,5,5' ,5'-octamethyl-2,2'-bi(1,3,2-dioxaborolane) (4,4,4',4',5,5,5',5'-octamethyl-2 ,2'-bi(1,3,2-dioxaborolane), 44.40 g, 174.8 mmol), potassium acetate (KOAc, 26 g) and Pd(dppf)Cl2 (2 g) in a nitrogen atmosphere N,N'-dimethylformamide(DMF, 400 mL). The reaction was stirred at 80° C. for 24 hours.Thereafter, the mixture was cooled to room temperature (25° C.) and extracted with ethyl acetate and water to obtain an organic layer. The remaining moisture was removed using sodium sulfate (Na2SO4), and the solvent was removed under reduced pressure and purified through column chromatography to obtain a white solid compound a-1 (22.9 g, yield: 95%).
References:
KR2021/14859,2021,A Location in patent:Paragraph 0141-0145

68867-14-1
48 suppliers
$31.60/1g

791614-90-9
17 suppliers
inquiry
93933-49-4
31 suppliers
inquiry

791614-90-9
17 suppliers
inquiry