
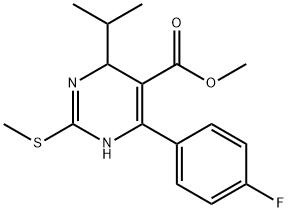
Methyl 4-(4-Fluorophenyl)-6-isopropyl-2-(methylsulfonyl)pyrimidine- 5-carboxylate synthesis
- Product Name:Methyl 4-(4-Fluorophenyl)-6-isopropyl-2-(methylsulfonyl)pyrimidine- 5-carboxylate
- CAS Number:799842-06-1
- Molecular formula:C16H17FN2O4S
- Molecular Weight:352.38

160009-35-8
10 suppliers
$140.00/25mg

799842-06-1
50 suppliers
$160.00/10mg
Yield:799842-06-1 90.92%
Reaction Conditions:
with ammonium peroxomolybdate;dihydrogen peroxide;Aliquat 336 in dichloromethane;water at 20 - 35; for 19 - 21.5 h;Product distribution / selectivity;
Steps:
1.B; 3.B; II
Step -B: PREPARATION OF METHYL-4- (4-FLUOROPHENYL)-6-ISOPROPYL-2-METHYL SULFONYL PYRIMIDINE-5-CARBOXYLATE (FORMULA VII) To a four neck clean RB flask, charged dichloromethane (1500.0 ml), Methyl -4- (4-fluorophenyl)-6-isopropyl-2-methylthio-pyrimidine-5-carboxylate (150.0gm,0.468 moles),ammonium heptamolybdate tetrahydrate (8.62 gm,0.00697 moles) and tricaprylyl methyl ammonium chloride (Aliquat336) (21.0 gm,0.047 moles) were stirred at 25 to 300C. This was followed by the slow addition of 50 % hydrogen peroxide solution (96.5 gm, 1.42 moles) at 20 to 350C over period of 1 to 1.5 hrs. Reaction mass were further stirred at 25 to 30 C for 18 to 20 hours. After the completion of reaction charged water (450.0 ml) and continued stirring for 10 min. This was followed by layer separation. The aqueous layer was extracted with 130.0 ml dichloromethane. Combined all organic layers and Washed with water (2 x 500.0 ml). Dichloromethane was distilled under reduced pressure at 40 to 450C and followed by degassing for 30 min at 50 to 550C under reduced pressure. Isopropyl alcohol (175.0 ml) was added and distilled out under vacuum at 50 to 55 0C, followed by the addition of isopropyl alcohol (350.0 ml) and heat to 60 to 650C to get clear solution. Cooled solution to 25 to 300C and stirred for 2.0 hrs at 25 to 300C .Further cooled to 0 to 5 0C and stirred for 2.0 hrs at 0 to 5 0C. Filtered and washed with chilled (0 0C) isopropyl alcohol (100.0 ml). The wet product was dried at 40 to 450C
References:
WO2008/59519,2008,A2 Location in patent:Page/Page column 8; 15-16; 20
885100-76-5
12 suppliers
inquiry

799842-06-1
50 suppliers
$160.00/10mg

459-57-4
582 suppliers
$6.00/25g

799842-06-1
50 suppliers
$160.00/10mg

42558-54-3
379 suppliers
$10.00/5g

799842-06-1
50 suppliers
$160.00/10mg

122549-26-2
24 suppliers
$95.00/50mg

799842-06-1
50 suppliers
$160.00/10mg