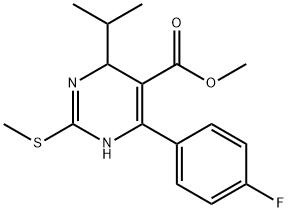
Methyl 6-(4-Fluorophenyl)-4-isopropyl-2-methylthio-1,4-dihydropyrimidine -5-carboxylate synthesis
- Product Name:Methyl 6-(4-Fluorophenyl)-4-isopropyl-2-methylthio-1,4-dihydropyrimidine -5-carboxylate
- CAS Number:885100-76-5
- Molecular formula:C16H19FN2O2S
- Molecular Weight:322.4

122549-26-2
23 suppliers
$95.00/50mg
867-44-7
371 suppliers
$5.00/10g

885100-76-5
11 suppliers
inquiry
Yield:885100-76-5 49%
Reaction Conditions:
with triethylamine in dimethyl sulfoxide at 25 - 75; for 17 h;
Steps:
3
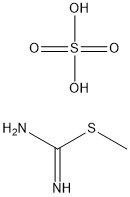
Example-3; Preparation of Methyl 6-(4-fluorophenyl)-4-isopropyl-2-(Methylthio)-l,4-dihydropyrimidine-5-carboxylate; In a round bottom flask equipped with stirrer, hot plate, water bath, condenser , thermometer, charge 1800 ml of dimethyl sulfoxide at 25 to 35 0C. Add 2-[(4-fluorophenyl) methylene-4 methyl-3-oxo pentanoic acid methyl ester (60Og, 2.40 mol), S-Methyl isothiourea sulphate (373.2g, 1.3424 mol), and triethylamine (147.6g, 1.4588 mol). Stir the mass. Raise the temperature between 7O0C to75 0C & maintain for 17 hrs. Check TLC for product formation. Cool the reaction mass to 10 to 150C. Add HCl solution (3036 ml of demineralised water & 480 ml of cone. HCl). Extract this aq. layer twice with 1020 ml toluene. Separate layers and discard toluene layer. To aqueous layer, add 780 ml of toluene into the flask at 25 to 35 0C. Add 882 ml of 20% aqueous ammonia slowly. Stir and settle for 30 minutes. Separate the aqueous layer and preserve toluene layer. Extract aqueous layer with 510 ml toluene separate layers and combine organic layer with main organic layer. Wash organic layer with 630 ml water. Dry this organic layer over sodium sulfate. Unload & weigh the organic layer. Volume of organic layer containing product = 1710 ml (1.5630 kg) Yield: 49%; purity by HPLC- NLT 80%
References:
WO2007/74391,2007,A2 Location in patent:Page/Page column 14

459-57-4
598 suppliers
$6.00/25g

885100-76-5
11 suppliers
inquiry

42558-54-3
390 suppliers
$10.00/5g

885100-76-5
11 suppliers
inquiry