
7H-pyrrolo[2,3-c]pyridazin-4-ol synthesis
- Product Name:7H-pyrrolo[2,3-c]pyridazin-4-ol
- CAS Number:1269822-97-0
- Molecular formula:C6H5N3O
- Molecular Weight:135.12
![4-hydroxy-7H-pyrrolo[2,3-c]pyridazine-6-carboxylic acid](/CAS/GIF/1269822-96-9.gif)
1269822-96-9
12 suppliers
inquiry
![7H-pyrrolo[2,3-c]pyridazin-4-ol](/CAS/GIF/1269822-97-0.gif)
1269822-97-0
17 suppliers
inquiry
Yield:1269822-97-0 77%
Reaction Conditions:
with sulfolane at 270; for 0.25 h;
Steps:
6.2 Synthesis of 7H-pyrrolo[2,3-c]pyridazin-4-ol (C21).
Tetrahydrothiophene 1,1-dioxide (sulfolane, 50 mL) was heated to 270 °C. Compound C20 (13.0 g, 72.6 mmol) was added portion-wise to the hot solvent; thereaction mixture was maintained at 270 °C for 15 minutes, then immediately cooled to room temperature. Purification via silica gel chromatography (Gradient: 1% to 17% methanol in dichloromethane) afforded the product as a yellow solid. Yield: 7.5 g, 56 mmol, 77%. 1H NMR (400 MHz, DMSO-d6) 12.34 (brs, 1H), 8.10 (brs, 1H), 7.51 (brs, I H), 6.63 (d, J=3.3 Hz, I H).
References:
WO2015/92592,2015,A1 Location in patent:Page/Page column 54; 55
![methyl 4-hydroxy-7H-pyrrolo[2,3-c]pyridazine-6-carboxylate](/CAS/GIF/1269822-95-8.gif)
1269822-95-8
12 suppliers
inquiry
![7H-pyrrolo[2,3-c]pyridazin-4-ol](/CAS/GIF/1269822-97-0.gif)
1269822-97-0
17 suppliers
inquiry
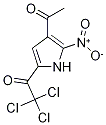
1269822-93-6
6 suppliers
inquiry
![7H-pyrrolo[2,3-c]pyridazin-4-ol](/CAS/GIF/1269822-97-0.gif)
1269822-97-0
17 suppliers
inquiry

1269822-94-7
7 suppliers
inquiry
![7H-pyrrolo[2,3-c]pyridazin-4-ol](/CAS/GIF/1269822-97-0.gif)
1269822-97-0
17 suppliers
inquiry
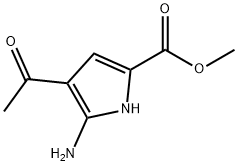
1269824-46-5
8 suppliers
inquiry
![7H-pyrrolo[2,3-c]pyridazin-4-ol](/CAS/GIF/1269822-97-0.gif)
1269822-97-0
17 suppliers
inquiry