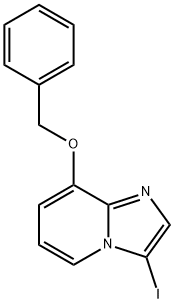
8-BENZYLOXY-3-IODO-IMIDAZO[1,2-A]PYRIDINE synthesis
- Product Name:8-BENZYLOXY-3-IODO-IMIDAZO[1,2-A]PYRIDINE
- CAS Number:885276-38-0
- Molecular formula:C14H11IN2O
- Molecular Weight:350.15
![8-BENZYLOXY-IMIDAZO[1,2-A]PYRIDINE](/CAS/GIF/96428-16-9.gif)
96428-16-9
41 suppliers
$60.00/100mg
![8-BENZYLOXY-3-IODO-IMIDAZO[1,2-A]PYRIDINE](/CAS/GIF/885276-38-0.gif)
885276-38-0
22 suppliers
$298.00/500mg
Yield:885276-38-0 83%
Reaction Conditions:
with N-iodo-succinimide in acetonitrile at 0 - 20;
Steps:
20
Example 20 8-benzyloxy-3-iodo-imidazo^ ,2-α]pyridine (31)As shown in Fig. 6, N-iodosuccinimide (7.0 g, 31.2 mmol) was added to a solution of 8-benzyloxy-imidazo[1 ,2-α]pyridine (Example 19) 24 (7.0 g, 31.2 mmol) in dry acetonitrile (100 ml.) at O0C. The reaction was warmed to room temperature and then stirred for 12 hours. The mixture was filtered and the cake washed with acetonitrile and water and dried under high vacuum to afford 8-benzyloxy-3-iodo-imidazo[1 ,2-α]pyridine 31 as colorless solid. 9.0 g, 83%, yield.1H NMR (300 MHz, CDCI3, 297K) δ 5.11 (s, 2H), 6.33 (d, J = 7.5 Hz, 1 H), 6.55 (t, J = 7.35 Hz, 1 H), 7.13 (m, 3H), 7.28 (d, J = 6.9 Hz, 2H), 7.45 (s, 1 H), 7.54 (d, J = 6.6 Hz, 1 H).13C NMR (75.5 MHz, CDCI3, 297K) δ:70.89, 103.23, 1 12.95, 119.15, 127.44, 128.18, 128.67, 135.98, 139.41 , 142.16, 147.84.ESI MS (MeOH) calcd for C14H11IN2O 350.16, found m/z (M++1 ) 351.
References:
WO2010/33978,2010,A2 Location in patent:Page/Page column 36