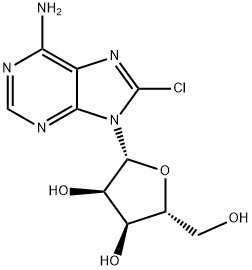
8-Chloroadenosine synthesis
- Product Name:8-Chloroadenosine
- CAS Number:34408-14-5
- Molecular formula:C10H12ClN5O4
- Molecular Weight:301.69

58-61-7
859 suppliers
$9.00/1g

34408-14-5
85 suppliers
$60.00/5 mg
Yield: 52%
Reaction Conditions:
with N-chloro-succinimide;acetic acid in N,N-dimethyl-formamide at 20; for 48 h;Concentration;Reagent/catalyst;
Steps:
1 8-Chioroadenosine
Procedure 2: Adenosine (1.09g, 4.08 mmol) was suspended in DMF (50 mL) and glacial acetic acid (10 mL) was added. A solution of N-chlorosuccinimide (2 g, 15 mmol) in DMF (15 mL) was added dropwise. The reaction mixture was stirred at rt for 48 hours and the volatiles were evaporated in vacuo to give yellow gum. The crude was absorbed on silica and purified by silica gel CC (packed in 5% MeOHICHC13, eluted with 7% MeOHICHC13) to yield a white powder (0.65 g, 52 %).‘H NIVIR (500 MHz, DMSO-d6) 8.16 (s, 1H, H-2), 7.55 (br s, 2H, NH2), 5.86 (d, J = 6.8 Hz, 1H, Hi’), 5.48 (d, J 6.2 Hz, iH, 2’OH), 5.45 (d, J 4.0 Hz, iH, 5’-OH), 5.23 (d, J4.6 Hz, iH, 3’OH), 5.08-5.06 (m, iH, H-2’), 4.23-4.i8 (m, iH, H3’), 4.01-3.96 (m, iH, H4’),3.72-3.65 (m, iH, H5’), 3.57-3.50 (m, iH, H5’).
References:
NUCANA BIOMED LIMITED;GRIFFITH, Hugh;SERPI, Michaela;SLUSARCZYK, Magdalena WO2017/207989, 2017, A1 Location in patent:Page/Page column 55; 56
4294-16-0
126 suppliers
$28.00/250mg

34408-14-5
85 suppliers
$60.00/5 mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
3001-45-4
28 suppliers
inquiry

34408-14-5
85 suppliers
$60.00/5 mg
2946-39-6
157 suppliers
$14.00/100mg

34408-14-5
85 suppliers
$60.00/5 mg