
7,9-DIMETHOXYCARBONYL-2-ETHOXYCARBONYL-1H-PYRROLO-[2,3-F]QUINOLINE-4,5-DIONE synthesis
- Product Name:7,9-DIMETHOXYCARBONYL-2-ETHOXYCARBONYL-1H-PYRROLO-[2,3-F]QUINOLINE-4,5-DIONE
- CAS Number:80721-47-7
- Molecular formula:C18H14N2O8
- Molecular Weight:386.31
![7,9-DIMETHOXYCARBONYL-2-ETHOXYCARBONYL-5-METHOXY-1H-PYRROLO-[2,3-F]QUINOLINE](/StructureFile/ChemBookStructure22/GIF/CB1648887.gif)
133706-80-6
12 suppliers
inquiry
![7,9-DIMETHOXYCARBONYL-2-ETHOXYCARBONYL-1H-PYRROLO-[2,3-F]QUINOLINE-4,5-DIONE](/StructureFile/ChemBookStructure22/GIF/CB8648886.gif)
80721-47-7
15 suppliers
$432.00/5mg
Yield:80721-47-7 62%
Reaction Conditions:
with ammonium cerium (IV) nitrate;water in acetonitrile at -5; for 1.58333 h;Cooling with acetone-dry ice;
Steps:
1.I; i
Into a 3-L, 3-neck flask equipped with a mechanical stirrer, a temperature probe, an addition funnel, and a dry ice / acetone bath were placed 5-methoxy-lH pyrrolo[2,3- f]qumoline-2,7,9-tricarboxylic acid 2-ethyl ester 7,9-dimethyl ester (9) (12.09 g, 0.0313 mol) and 600 mL of acetonitrile. The suspension was stirred and cooled to -5 0C. A solution of cerric ammonium nitrate (84.87 g, 0.155 mol) in 120 mL of water was added over a five- minute period. A bright orange solution resulted. Stirring and cooling at -5 0C was continued for 1.5 hours. The reaction mixture was poured into 1380 mL of vigorously-stirred, cold water and stirring was continued for 0.5 hour. The cerrium salts were filtered off and the filtrate was extracted with 3 times with 300 mL of methylene chloride. The methylene chloride solution was dried over 75 g of magnesium sulfate and the solvent removed in vacuo to producel0.15 g of solid. The resulting solid was stirred in a solution of 10 mL of toluene and 10 mL of ethyl acetate. The resulting bright red-orange crystals were collected by filtration and washed with a solution of 3 : 1 ethyl acetate - heptane. The crystals were taken up in 360 mL of methylene chloride and stirred with 5 g of silica gel for one hour. The solution was filtered through Celite 521 and stripped to 7.50 g (62.0%) of the title compound as a red-orange solid.; In step i, 5-methoxy-lH-pyrrolo[2,3-fJquinoline-2,7,9-tricarboxylic acid 2-ethyl ester 7,9-dimethyl ester (9) and acetonitrile were placed in a reaction vessel, cooled and stirred. To this suspension was added a solution of aqueous cerric ammonium nitrate ((NBU)2Ce(NOs)6 or CAN) to produce a bright orange solution which was then poured into cold water. The cerrium salts were collected under vacuum and the filtrate was extracted with CH2Cl2. The organic extracts were dried over magnesium sulfate (MgSO4) and concentrated. The resulting residue was taken up in a solution of toluene-ethyl acetate (EtOAc), and the resulting crystals were collected by filtration, washed with EtO Ac-heptane, taken up in CH2Cl2 and stirred with silica gel for 1 hour, and then filtered through Celite. The solvent was removed to produce 4,5-dioxo-4,5-dihydro-l H-pyrrolo[2,3-f]quinoline-2,7,9- tricarboxylic acid 2-ethyl ester 7,9-dimethyl ester (10).
References:
WO2006/102642,2006,A1 Location in patent:Page/Page column 10; 17; 1/6
![5H-Indole-7-acetic acid, 6-chloro-α-[2,3-dihydro-4-(methoxycarbonyl)-2-oxo-5-oxazolyl]-2-(ethoxycarbonyl)-4-hydroxy-5-oxo-, methyl ester](/CAS/20210305/GIF/98126-30-8.gif)
98126-30-8
0 suppliers
inquiry
![7,9-DIMETHOXYCARBONYL-2-ETHOXYCARBONYL-1H-PYRROLO-[2,3-F]QUINOLINE-4,5-DIONE](/StructureFile/ChemBookStructure22/GIF/CB8648886.gif)
80721-47-7
15 suppliers
$432.00/5mg
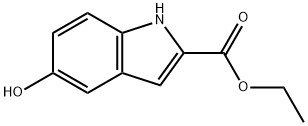
24985-85-1
133 suppliers
$19.00/100mg
![7,9-DIMETHOXYCARBONYL-2-ETHOXYCARBONYL-1H-PYRROLO-[2,3-F]QUINOLINE-4,5-DIONE](/StructureFile/ChemBookStructure22/GIF/CB8648886.gif)
80721-47-7
15 suppliers
$432.00/5mg
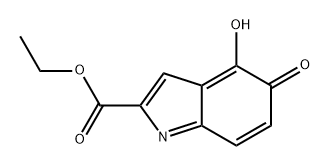
98126-24-0
0 suppliers
inquiry
![7,9-DIMETHOXYCARBONYL-2-ETHOXYCARBONYL-1H-PYRROLO-[2,3-F]QUINOLINE-4,5-DIONE](/StructureFile/ChemBookStructure22/GIF/CB8648886.gif)
80721-47-7
15 suppliers
$432.00/5mg
![Glutamic acid, 4-nitro-N-[(phenylmethoxy)carbonyl]-, dimethyl ester (9CI)](/CAS/20210305/GIF/204922-65-6.gif)
204922-65-6
0 suppliers
inquiry
![7,9-DIMETHOXYCARBONYL-2-ETHOXYCARBONYL-1H-PYRROLO-[2,3-F]QUINOLINE-4,5-DIONE](/StructureFile/ChemBookStructure22/GIF/CB8648886.gif)
80721-47-7
15 suppliers
$432.00/5mg