
N,N-DIETHYL-1,5-DIHYDRO-2,4,3-BENZODIOXAPHOSPHEPIN-3-AMINE synthesis
- Product Name:N,N-DIETHYL-1,5-DIHYDRO-2,4,3-BENZODIOXAPHOSPHEPIN-3-AMINE
- CAS Number:82372-35-8
- Molecular formula:C12H18NO2P
- Molecular Weight:239.25
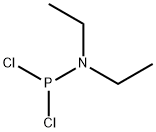
1069-08-5
122 suppliers
$20.00/5g
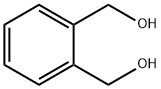
612-14-6
172 suppliers
$10.00/1g

82372-35-8
41 suppliers
$100.00/250 mg
Yield:82372-35-8 100%
Reaction Conditions:
with diethylamine in tetrahydrofuran at -78 - 20; for 18 h;
Steps:
N,N-Diethyl-1,5-dihydrobenzo[e][1,3,2]dioxaphosphepin-3-amine (13)
1,1-Dichloro-N,N-diethylphosphinamine (2.52 g, 14.5 mmol) was subsequentlydissolved in dry ether (100 mL) and cooled to -78 C. A solution of triethylamine(5.86 g, 8.0 mL, 29.0 mmol) and phenyldimethanol (2.0 g, 14.5 mmol) dissolved in amixture of dry ether (80 mL) and dry THF (20 mL) was prepared and added to thestirring reaction mixture via cannula. After 1 hour of stirring at -78 C the reactionmixture was slowly allowed to warm to room temperature and was stirred for a further17 hours. The resulting precipitate was removed by Schlenk filtration and washed withmore dry ether before being reduced to dryness under inert atmosphere to yield thetitle compound 13 (3.5 g, quant.) as a clear oil. The product was dissolved in dry dichloromethane at a concentration of ~1.02 M and stored in a Schlenk flask to reducethe likelihood of oxidation H (500 MHz; CDCl3) 7.26-7.17 (4H, m), 5.16 (2H, dd, J = 13.8, 6.9 Hz), 4.89 (2H,dd, J = 19.5, 13.8 Hz), 3.17 (2H, q, J = 7.1 Hz), 3.15 (2H, q, J = 7.1), 1.09 (6H, t, J =7.1 Hz); P (202 MHz; CDCl3) 145.29. This data corresponds well with previouslyreported data.308 The compound was not characterised further
References:
Gregory, Mark;Catimel, Bruno;Yin, Meng-Xin;Condron, Melanie;Burgess, Antony W.;Holmes, Andrew B. [Synlett,2016,vol. 27,# 1,art. no. ST-2015-D0764-L,p. 121 - 125] Location in patent:supporting information

612-14-6
172 suppliers
$10.00/1g
2283-11-6
171 suppliers
$30.00/1g

82372-35-8
41 suppliers
$100.00/250 mg