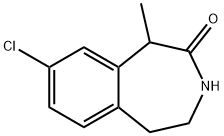
2H-3-Benzazepin-2-one, 8-chloro-1,3,4,5-tetrahydro-1-Methyl- synthesis
- Product Name:2H-3-Benzazepin-2-one, 8-chloro-1,3,4,5-tetrahydro-1-Methyl-
- CAS Number:824430-77-5
- Molecular formula:C11H12ClNO
- Molecular Weight:209.67
![2-CHLORO-N-[2-(4-CHLORO-PHENYL)-ETHYL]-PROPIONAMIDE](/CAS/GIF/34164-14-2.gif)
34164-14-2
14 suppliers
inquiry

824430-77-5
22 suppliers
inquiry
Yield:824430-77-5 54%
Reaction Conditions:
with aluminum (III) chloride at 150; for 12 h;
Steps:
3.7.2 Step2: Preparation of 8-dichloro-1-methyl-2,3,5-trihydro-1H-3-benzazepin-2-one.
2- (4-CHLOROPHENYL) ethyl-N-2-chloropropionamide (10 g, 40.6 mmol) and aluminum chloride (16 g, 119.9 mmol) were added to a clean dry 100 ML round bottom flask equipped with an argon balloon, stirring apparatus, and heating apparatus. The white solid melted to a tan oil with bubbling at 91 °C. (Note: if impure starting materials are used, a black tar can result but clean product can still be isolated). The mixture was heated and stirred at 150°C for 12 hours. The reaction can be followed by LC/MS with the starting material at 2.45 minutes (246.1 M++H), the product at 2.24 minutes (209.6 M++H+) on a 5 minute reaction time from 5-95% W/0. 01 % TFA in water/MeCN (50: 50). After cooling to room temperature, the reaction mixture was quenched with slow addition of 10 ML OF MEOH followed by 5 mL of 5 % HC1 in water and 5 ML of ethyl acetate. After separation of the resulting layers, the aqueous layer was extracted a second time with 10 mL of ethyl acetate. The combined organic layers were dried over magnesium sulfate, filtered, and concentrated to a tan solid (6.78 grams, 80 % yield). LC/MS showed one peak, at 2.2 min and 209.6 MI. This material was taken up in ethyl acetate, filtered through celite and Kieselgel 60 (0.5 inch plug on a 60 mL Buchner funnel) and the filtrate was recrystallized from hexane/ethyl acetate to give final product (4.61 grams, 54 % YIELD). H NMR (CDC13) : 8 7.3-7. 1 (M, 3H, Ar), 5.6 (br s, 1H, NH), 4.23 (q, 1H, CHCH3), 3.8 (M, 1H, ARCH2CH2NH), 3.49 (M, 1H, ARCH2CH2NH), 3.48 (M, 1H, ARC_2CH2NH), 3.05 (m, 1H, ARCH2CH2NH), 1.6 (d, 3H, CH2). 13C NMR (CDC13) : 178 (1C, C=O), 139 (1C, Ar), 135 (1C, Ar), 130, 129 (2C, Ar), 126 (2C, Ar), 42 (1C, C), 40 (1C, CHN), 33 (1C, CHAr), 14 (1C, CH3).
References:
WO2005/3096,2005,A1 Location in patent:Page 52
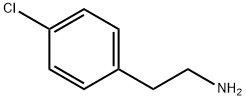
156-41-2
293 suppliers
$23.00/5g

824430-77-5
22 suppliers
inquiry