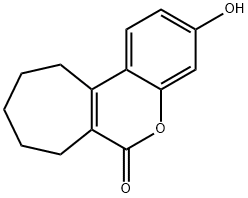
3-HYDROXY-8,9,10,11-TETRAHYDRO-7H-CYCLOHEPTACCHROMEN-6-ONE synthesis
- Product Name:3-HYDROXY-8,9,10,11-TETRAHYDRO-7H-CYCLOHEPTACCHROMEN-6-ONE
- CAS Number:83688-44-2
- Molecular formula:C14H14O3
- Molecular Weight:230.26
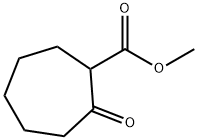
774-05-0
83 suppliers
$87.00/200MG
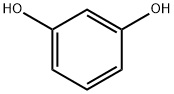
108-46-3
785 suppliers
$10.00/10g

83688-44-2
25 suppliers
inquiry
Yield: 78%
Reaction Conditions:
with methanesulfonic acid at 25; for 4 h;
Steps:
5.5.1.5
The first stage consists of condensing 2-carbetoxycycloheptanone with resorcinol in methanesulphonic acid, the reaction is taken to 25° C. for 4 hours. The intermediate 3-hydroxy-8,9,10,11-tetrahydrocyclohepta[c]chromen-6(7H)-one thus formed precipitates by adding ethanol then water then it is isolated by filtration, dried under vacuum at 60° C. and a yield of 78% is obtained. In a second stage, sulphonylisocyanate chloride is converted, in toluenic solution, to sulphamoyl chloride by the action of the formic acid, then condensed with the previous intermediate dissolved in N,N-dimethylacetamide (DMA). The reaction medium is treated with water and extracted with 2-methyltetrahydrofurane (2-MeTHF). The crude compound 1 is then obtained by filtration of the precipitate obtained by adding methylcyclohexane to the organic phase. Finally, pure compound 1 is obtained by recrystallization of the crude product by dissolution in acetone while hot and precipitation by adding methylcyclohexane (the addition of the antisolvent methylcyclohexane having the main purpose of increasing the yield).Compound 1 is thus obtained with a yield of 65%, and presented hereafter.Example 1 thus obtained can be subjected to an appropriate treatment aimed at reducing the size of the particles such as micronization (point 5.3 below) or wet grinding (point 5. below).
References:
Ipsen Pharma S.A.S. US2012/321714, 2012, A1 Location in patent:Page/Page column 12

502-42-1
216 suppliers
$10.00/1g

83688-44-2
25 suppliers
inquiry