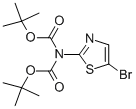
N-(5-BROMOTHIAZOL-2-YL)ZAZBIS(BISCARBONIC ACID BIS-1,1-DIMETHYLETHYL ESTER synthesis
- Product Name:N-(5-BROMOTHIAZOL-2-YL)ZAZBIS(BISCARBONIC ACID BIS-1,1-DIMETHYLETHYL ESTER
- CAS Number:840493-96-1
- Molecular formula:
- Molecular Weight:0

24424-99-5
820 suppliers
$13.50/25G
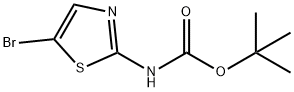
405939-39-1
166 suppliers
$14.00/1g

840493-96-1
4 suppliers
inquiry
Yield:840493-96-1 85%
Reaction Conditions:
with dmap;triethylamine in tetrahydrofuran at 0 - 20;
Steps:
1.iii-vi
Di-tert-butyl dicarbonate [(Boc)20, 90.4 g, 0.414 mol, 1.2 eq] was added to a flask containing a mixture of intermediate lb’ (97.9 g, 0.35 1 mol, 1.0 eq) and 4- (dimethylamino)pyridine (DMAP, 1.07 g, 8.7 mmol, 0.025 eq) in 880 ml of THF and 121 ml of Et3N and cooled to 0 °C using an ice bath. The reaction mixture was stirred at rt overnight. Water (200 ml) and DCM were added and the resulting mixture was stirred at rt for 30 mm. The organic layer was separated and the aqueous layer was extracted with DCM (2 x 150 ml). The combined organic layers were washed with brine, dried over Na2SO4, filtered and concentrated in vacuo. The crude residue was purified with a short silica gel column (eluting with EtOAc/Heptane 1:20) to give intermediate lc’ as an off-white solid (113 g, 85% yield).
References:
WO2018/112136,2018,A1 Location in patent:Paragraph 00262

24424-99-5
820 suppliers
$13.50/25G

840493-96-1
4 suppliers
inquiry
1009628-15-2
0 suppliers
inquiry

840493-96-1
4 suppliers
inquiry
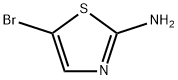
3034-22-8
210 suppliers
$7.00/250mg

24424-99-5
820 suppliers
$13.50/25G

840493-96-1
4 suppliers
inquiry