
Trifluoro-methanesulfonic acid 4-tert-butoxycarbonylamino-cyclohex-1-enyl ester synthesis
- Product Name:Trifluoro-methanesulfonic acid 4-tert-butoxycarbonylamino-cyclohex-1-enyl ester
- CAS Number:849069-49-4
- Molecular formula:C12H18F3NO5S
- Molecular Weight:345.34
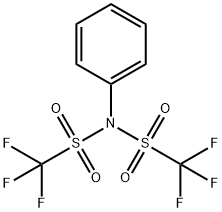
37595-74-7
425 suppliers
$5.00/1g
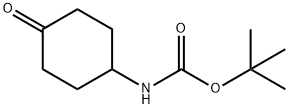
179321-49-4
305 suppliers
$5.00/1g

849069-49-4
19 suppliers
$393.00/250mg
Yield:849069-49-4 71.6%
Reaction Conditions:
Stage #1: N-(tert-butoxycarbonyl)-4-aminocyclohexanonewith lithium hexamethyldisilazane in tetrahydrofuran at -78; for 1 h;Inert atmosphere;
Stage #2: N,N-phenylbistrifluoromethane-sulfonimide in tetrahydrofuran at 15; for 16 h;Inert atmosphere;
Steps:
48A Example 48A 4-((tert-butoxycarbonyl)amino)cyclohex-1-en-1-yl trifluoromethanesulfonate
Example 48A
4-((tert-butoxycarbonyl)amino)cyclohex-1-en-1-yl trifluoromethanesulfonate
To a solution of tert-butyl (4-oxocyclohexyl)carbamate (1 g, 4.689 mol) in THF (20 mL) was added dropwise LiHMDS (1 M, 9.4 ml, 9.378 mmol) at -78° C. under N2 atmosphere.
After stirring at -78° C. for 1 h, a solution of (CF3SO2)2NPh (1.84 g, 5.158 mmol) in THF (5 mL) was added.
The mixture was stirred at 15° C. for 16 h, and then quenched with aq NH4Cl solution (20 mL).
The aqueous layer was extracted with EtOAc (20 mL*2).
The combined organic layers were washed with water, brine, dried over Na2SO4, filtered and evaporated.
The residue was purified by column chromatograph on silica gel to provide the title compound as a white solid (1.16 g, yield: 71.60%). LCMS (ESI) m/z: 347 (M+1).
References:
US2017/29430,2017,A1 Location in patent:Paragraph 0479

37595-74-7
425 suppliers
$5.00/1g

849069-49-4
19 suppliers
$393.00/250mg
![2-[N,N-BIS(TRIFLUOROMETHANESULFONYL)AMINO]-5-CHLOROPYRIDINE](/CAS/GIF/145100-51-2.gif)
145100-51-2
218 suppliers
$6.00/1g

179321-49-4
305 suppliers
$5.00/1g

849069-49-4
19 suppliers
$393.00/250mg
111300-06-2
186 suppliers
$6.00/1g

849069-49-4
19 suppliers
$393.00/250mg
27489-62-9
456 suppliers
$5.00/5g

849069-49-4
19 suppliers
$393.00/250mg