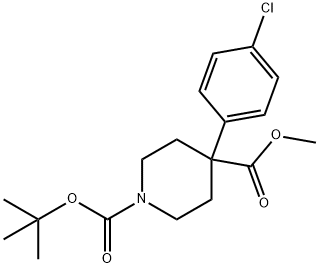
1-BOC-4-(4-CHLOROPHENYL)-4-PIPERIDINEDICARBOXYLIC ACID METHYL ESTER synthesis
- Product Name:1-BOC-4-(4-CHLOROPHENYL)-4-PIPERIDINEDICARBOXYLIC ACID METHYL ESTER
- CAS Number:849106-01-0
- Molecular formula:C18H24ClNO4
- Molecular Weight:353.84
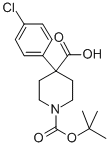
644981-94-2
31 suppliers
$70.00/50mg
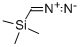
18107-18-1
208 suppliers
$33.00/5g

849106-01-0
5 suppliers
inquiry
Yield:849106-01-0 335 mg
Reaction Conditions:
in methanol at 20; for 0.5 h;
Steps:
44.A Step A: 1-tert-Butyl 4-methyl 4-(4-chlorophenyl)piperi dine- 1,4- dicarboxylate:
to a slotion of l-(tert-butoxycarbonyl)-4-(4-chlorophenyl)piperidine-4- carboxylic acid (296 mg, 0.871 mmol) in methanol (2904 μ) at rt was added (0194) trimethylsilyl diazomethane (871 μ, 1.742 mmol), and the reaction mixture was stirred at rt for 30 min. Methanol was removed in vacuo, water was added and the aqueous layer was extracted with ethyl acetate (x3). The combined organic layers were washed with brine, dried over anhydrous sodium sulfate, and filtered, and the filltrate was evaporated in vacuo to give 1-tert-butyl 4-methyl 4-(4-chlorophenyl)piperi dine- 1 ,4-dicarboxy late (335 mg) as a yellow oil. This material was used directly for the reduction reaction. NMR (500MHz, CHLOROFORM-d) δ 7.35 - 7.29 (m, 4H), 3.97 (br. s., 2H), 3.69 (s, 3H), 3.02 (br. s., 2H), 2.51 (d, J=13.1 Hz, 2H), 1.83 (br. s., 2H), 1.47 (s, 9H).
References:
WO2018/81384,2018,A1 Location in patent:Page/Page column 56

644981-94-2
31 suppliers
$70.00/50mg

74-88-4
342 suppliers
$15.00/10g

849106-01-0
5 suppliers
inquiry

118753-70-1
177 suppliers
$6.00/1g

849106-01-0
5 suppliers
inquiry
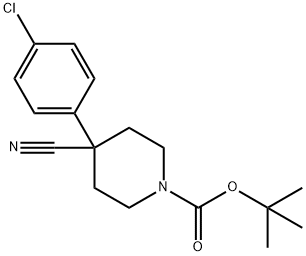
218451-34-4
31 suppliers
inquiry

849106-01-0
5 suppliers
inquiry

24424-99-5
823 suppliers
$13.50/25G

849106-01-0
5 suppliers
inquiry