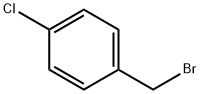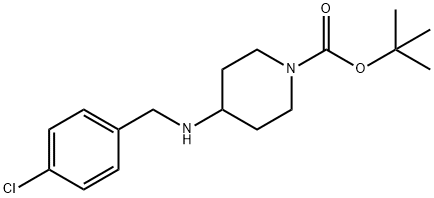
tert-Butyl 4-(4-chlorobenzylamino)piperidine-1-carboxylate synthesis
- Product Name:tert-Butyl 4-(4-chlorobenzylamino)piperidine-1-carboxylate
- CAS Number:849106-37-2
- Molecular formula:C17H25ClN2O2
- Molecular Weight:324.85

79099-07-3
543 suppliers
$5.00/5g

104-86-9
280 suppliers
$6.00/5g

849106-37-2
7 suppliers
$292.00/1g
Yield:849106-37-2 100%
Reaction Conditions:
with sodium tris(acetoxy)borohydride;acetic acid in 1,2-dichloro-ethane;
Steps:
500.1 Step 1.
Step 1. To a solution of 4-chlorobenzylamine (859 mg, 6.07 mmol) in 1,2-dichloroethane (20 mL) was added t-butyl-4-oxo-piperidine-1-carboxylate (1.451 g, 7.282 mmol), acetic acid (400 uL), and sodium triacetoxyborohydride (1.800 g, 8.497 mmol). The mixture was stirred at rt under nitrogen for 2 h, diluted with ethyl acetate, washed thrice with saturated sodium bicarbonate solution and dried over magnesium sulfate. The extracts were filtered and concentrated to afford 4-(4-chloro-benzylamino)-piperidine-1-carboxylic acid tert-butyl ester (2.49 g, >100%) as a yellow oil, which was of sufficient purity by H-NMR to use without further purification. 1H-NMR (CDCl3, 300 MHz) δ: 1.44 (s, 9H), 1.56-1.70 (br s, 2H), 1.81-1. 90 (br d, 2H), 2.63 (dddd, 1H), 2.78 (dd, 2H), 3.72 (s, 1H), 3.78 (s, 1H), 4.00 (br s, 2H), 7.16-7.25 (m, 4H). MS m/z: 325 (M+1).
References:
US2005/70549,2005,A1
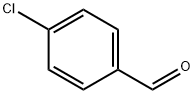
104-88-1
612 suppliers
$5.00/25g
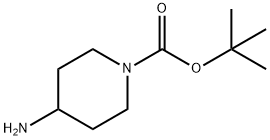
87120-72-7
20 suppliers
$6.00/1g

849106-37-2
7 suppliers
$292.00/1g