
(S)-tert-butyl 3-(isopropylamino)pyrrolidine-1-carboxylate synthesis
- Product Name:(S)-tert-butyl 3-(isopropylamino)pyrrolidine-1-carboxylate
- CAS Number:849107-00-2
- Molecular formula:C12H24N2O2
- Molecular Weight:228.33
![1-Pyrrolidinecarboxylic acid, 3-[(1-methylethylidene)amino]-, 1,1-dimethylethyl ester, (3R)-](/CAS/20210305/GIF/1061682-27-6.gif)
1061682-27-6
0 suppliers
inquiry

849107-00-2
19 suppliers
$403.00/1g
Yield:849107-00-2 53%
Reaction Conditions:
with hydrogen;platinum oxide in methanol; for 18 h;
Steps:
2.G Method G: (R)-tert-butyl 3-(isopropylamino)pyrrolidine-1-carboxylate
Method G: (R)-tert-butyl 3-(isopropylamino)pyrrolidine-1-carboxylate Platinum oxide (60 mg) was added to a solution of (R)-tert-butyl 3-(propan-2-ylideneamino)pyrrolidine-1-carboxylate (600 mg, 2.65 mmol) in methanol (4 ml). The reaction was stirred for 18 hours under an atmosphere of hydrogen. The reaction mixture was filtered through a path of celite and washed with more methanol. The filtrate was concentrated in vacuo to afford an oil which solidified on standing (320 mg, 53% yield). 1H NMR (CDCl3, 400 MHz) δ 1.02 (6H, d), 1.38 (9H, s), 1.58-1.72 (2H, m), 2.02 (1H, m), 2.82 (1H, m), 3.08 (1H, m), 3.20 (1H, m), 3.35 (1H, m), 3.40-3.60 (2H, m).
References:
US2010/137305,2010,A1 Location in patent:Page/Page column 31

67-64-1
6 suppliers
$21.60/10ml
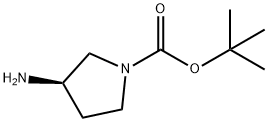
147081-49-0
321 suppliers
$10.00/1g

849107-00-2
19 suppliers
$403.00/1g