
(4-Fluoro-phenyl)-(3-hydroxy-piperidin-1-yl)-Methanone, 98+% C12H14FNO2, MW: 223.25 synthesis
- Product Name:(4-Fluoro-phenyl)-(3-hydroxy-piperidin-1-yl)-Methanone, 98+% C12H14FNO2, MW: 223.25
- CAS Number:851883-00-6
- Molecular formula:C12H14FNO2
- Molecular Weight:223.24
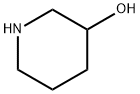
6859-99-0
399 suppliers
$6.00/5g
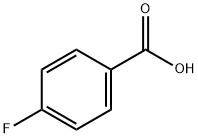
456-22-4
468 suppliers
$10.00/100g

851883-00-6
14 suppliers
$429.00/1g
Yield:851883-00-6 53%
Reaction Conditions:
with 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride;triethylamine;benzotriazol-1-ol in dichloromethane at 20;
Steps:
68(A)
Example 68 (4-Fluoro-phenyl)- [3- (5-phenyl-tetrazol-2-yl)-piperidin-1-yl]-methanone; 68 (A) (4-Fluoro-phenyl)- (3-hydroxy-piperidin-1-yl)-methanone A mixture of 3-hydroxypiperidine (0.6 g, 5.93 mmol), 4-fluorobenzoic acid (0. 83 g, 5.93 mmol), HOBT (0.8 g, 5.93 mmol), EDCI. HC1 (1.7 g, 8.9 mmol) and dry triethylamine (1.66 mL, 11.86 mmol) in dry dichloromethane (30 mL) was kept under stirring overnight at ambient temperature, under nitrogen atmosphere. HCl IN (30 mL) was then added and the phases separated. The organic layer was washed with HCl IN (30 mL), with NaOH IN (30 mL x 2 times), then with water (30 mL). Evaporation of the organic solvent afforded (4-fluoro-phenyl)- (3-hydroxy-piperidin- 1-yl)-methanone as a white oil (0.7 g). Yield: 53%; LCMS (Tr): 5.49 min (Method A); MS (ES+) gave m/z: 224.1. 'H-NMR (CDC13, 300 MHz), 8 (ppm): 7.43 (dd, 2H); 7.08 (dd, 2H); 3.83 (m, 1H); 3.73 (m, 1H); 3.56 (m, 1H); 3.43 (m, 2H); 1.99-1. 79 (m, 2H); 1.74-1. 42 (m, 2H).
References:
WO2005/44797,2005,A1 Location in patent:Page/Page column 98