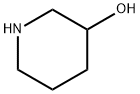
3-Hydroxypiperidine synthesis
- Product Name:3-Hydroxypiperidine
- CAS Number:6859-99-0
- Molecular formula:C5H11NO
- Molecular Weight:101.15
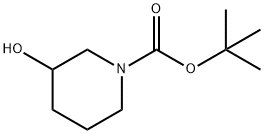
85275-45-2
368 suppliers
$7.00/5g

6859-99-0
404 suppliers
$6.00/5g
Yield:6859-99-0 450 mg
Reaction Conditions:
with hydrogenchloride in ethyl acetate at 20;
Steps:
164.2 Step 2: 2-Chloro-4-cyclopropyl-6-(3-hydroxypiperidin-1-yl)pyridine-3,5- dicarbonitrile
tert-Butyl 3-hydroxypiperidine-1-carboxya (1 g, 5 mmol) and HCl (2.0 M in EtOAc, 5 mL) were stirred at room temperature overnight. The solvent was removed under reduced pressure. The residue was neutralized by Sat. NaHCO3 (aq), and extracted with DCM. The organic layer was washed with brine, dried and purified by column chromatography, eluting with MeOH/DCM, 0-10%, to give piperidin-3-ol (450 mg).
References:
WO2017/216726,2017,A1 Location in patent:Page/Page column 734

109-00-2
586 suppliers
$10.00/10g

6859-99-0
404 suppliers
$6.00/5g
14813-01-5
331 suppliers
$9.00/1g

6859-99-0
404 suppliers
$6.00/5g

65520-06-1
0 suppliers
inquiry

6859-99-0
404 suppliers
$6.00/5g
4795-29-3
171 suppliers
$30.05/25ML

6859-99-0
404 suppliers
$6.00/5g