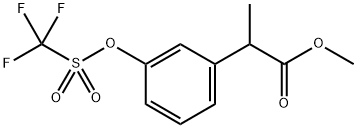
α-Methyl-3-[[(trifluoromethyl)sulfonyl]oxy]-benzeneacetic Acid Methyl Ester synthesis
- Product Name:α-Methyl-3-[[(trifluoromethyl)sulfonyl]oxy]-benzeneacetic Acid Methyl Ester
- CAS Number:859828-49-2
- Molecular formula:C11H11F3O5S
- Molecular Weight:312.26
Yield:859828-49-2 75%
Reaction Conditions:
Stage #1: methyl [3-[[(trifluoromethyl)sulfonyl]oxy]phenyl]-acetatewith lithium hexamethyldisilazane in tetrahydrofuran at -78; for 0.333333 h;Inert atmosphere;
Stage #2: methyl iodide in tetrahydrofuran at -78 - 20;Inert atmosphere;
Steps:
In a 250 ml round-bottomed flask lithium hexamethyldisilazide (1M in THF, 31.4 mL, 0.031 mol) and methyl(3-{ [trifluoromethyl)sulfonyl]oxy}phenyl)acetate (8.9 g, 0.03 mol) were dissolved in anhydrous THF (170 ml) under nitrogen atmosphere. After cooling to -78°C and stirring for 20 min, iodomethane (1.86 mL, 0.03 mol) was added dropwise. The resulting mixture was left warming up to room temperature and stirred overnight. An aqueous solution of ΚΗ2Ρ04 (30 ml) was added and the aqueous layer extracted with EtOAc (2 x 20 mL). The collected organic extracts were washed with water (2 x 20 ml), dried over anhydrous Na2S04 and evaporated under reduced pressure to give a crude residue that, after purification by flash chromatography (n-hexane/EtOAc 9: 1), afforded methyl 2-(3-{ [(trifluoromethyl)sulfonyl]oxy}phenyl)propanoate (7.5 g, 0.025 mol, 75% yield) as yellow oil used for the next step.
References:
WO2011/51375,2011,A1 Location in patent:Page/Page column 15
621-37-4
299 suppliers
$6.00/1g
![α-Methyl-3-[[(trifluoromethyl)sulfonyl]oxy]-benzeneacetic Acid Methyl Ester](/CAS/20210111/GIF/859828-49-2.gif)
859828-49-2
2 suppliers
inquiry
42058-59-3
113 suppliers
$15.00/1g
![α-Methyl-3-[[(trifluoromethyl)sulfonyl]oxy]-benzeneacetic Acid Methyl Ester](/CAS/20210111/GIF/859828-49-2.gif)
859828-49-2
2 suppliers
inquiry

