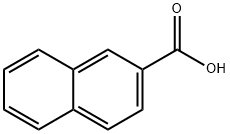
2-Naphthalenecarboxylic acid, 6-bromo-, ethyl ester synthesis
- Product Name:2-Naphthalenecarboxylic acid, 6-bromo-, ethyl ester
- CAS Number:86471-14-9
- Molecular formula:C13H11BrO2
- Molecular Weight:279.13
Yield:86471-14-9 97.5%
Reaction Conditions:
Stage #1: ethanol;naphthalene-2-carboxylate at 155; under 5320.36 Torr; for 1.16667 h;
Stage #2: with sodium hypobromite;benzenesulfonic acid;sodium bromide in water at 180; under 12920.9 Torr; for 7 h;
Steps:
3 A method for synthesizing ethyl 6-bromo-2-naphthoate, comprising the steps of:
Mix 2-naphthoic acid and ethanol, add catalyst, stir and pass argon gas, control the pressure to 7 atmospheres, control the temperature to 155 ° C, maintain 70 min; start mixing with the mixture of sodium bromide and benzenesulfonic acid In the aqueous solution, when the dropping volume reaches 10% of the volume of the solution, the aqueous solution of sodium hypobromite is added dropwise; the dropping rate of the two solutions is controlled to be the same, and the total dropping time is 150 min; after the completion of the dropwise addition, the temperature of the system is raised to 180 ° C. The pressure is raised to 17 atmospheres and the reaction is continued for 7 hours.The catalyst was obtained by the following method: the nano titanium dioxide and the nano silica were uniformly ground, soaked with hydrochloric acid having a mass concentration of 10% for 6 hours, filtered, washed with water, dried, and dried at 350 ° C.The molar ratio of sodium bromide to benzenesulfonic acid is 1:2.25; the molar ratio of 2-naphthoic acid to sodium bromide is 1:1.35; the molar ratio of sodium bromide to sodium hypobromite is 1:1; sodium bromide The molar concentration with sodium hypobromite was 2.2 mol/L.2) The solid was removed by hot filtration, and added to 5 times by weight of ice water of the filtrate, extracted with chloroform, dried over anhydrous sodium sulfate and concentrated to remove solvent to give ethyl 6-bromo-2-naphthoate.The molar yield was 97.5% and the GC purity was 98.3%.
References:
CN109438230,2019,A Location in patent:Paragraph 0040-0046
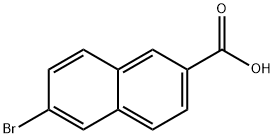
5773-80-8
306 suppliers
$10.00/1g

75-03-6
369 suppliers
$11.00/5g

86471-14-9
19 suppliers
$312.00/10g

64-17-5
825 suppliers
$10.00/50g

5773-80-8
306 suppliers
$10.00/1g

86471-14-9
19 suppliers
$312.00/10g

5773-80-8
306 suppliers
$10.00/1g

86471-14-9
19 suppliers
$312.00/10g
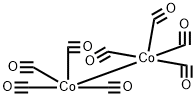
61117-58-6
0 suppliers
inquiry

64-17-5
825 suppliers
$10.00/50g
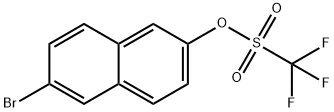
151600-02-1
59 suppliers
$23.00/250mg

86471-14-9
19 suppliers
$312.00/10g