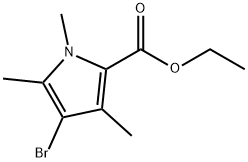
Ethyl 4-bromo-1,3,5-trimethyl-1H-pyrrole-2-carboxylate synthesis
- Product Name:Ethyl 4-bromo-1,3,5-trimethyl-1H-pyrrole-2-carboxylate
- CAS Number:86614-23-5
- Molecular formula:C10H14BrNO2
- Molecular Weight:260.13
5408-07-1
29 suppliers
$45.95/1gm:

74-88-4
348 suppliers
$15.00/10g

86614-23-5
7 suppliers
$134.31/250mgs:
Yield:86614-23-5 67.3%
Reaction Conditions:
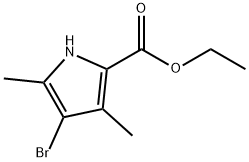
Stage #1: ethyl 4-bromo-3,5-dimethylpyrrole-2-carboxylatewith sodium hydride in tetrahydrofuran;mineral oil at 0; for 0.166667 h;
Stage #2: methyl iodide in tetrahydrofuran;mineral oil at 25; for 2 h;
Steps:
143.2 Step 2:
ethyl 4-bromo-1,3,5-trimethyl-pyrrole-2-carboxylate
A mixture of ethyl 4-bromo-3,5-dimethyl-1H-pyrrole-2-carboxylate (450 mg, 1.83 mmol) in tetrahydrofuran (20 mL) was stirred at 0° C. for 10 min.
Sodium hydride (60 mg, 2.5 mmol) was added at 0° C.
The reaction mixture was stirred at 0° C. for 10 min.
Iodomethane (312 mg, 2.2 mmol) was then added.
The reaction mixture was warmed to 25° C. and stirred for 2 h.
The reaction mixture was concentrated and purified by flash column chromatography (PE/EA=20/1) to give ethyl 4-bromo-1,3,5-trimethyl-pyrrole-2-carboxylate (320 mg, 1.23 mmol, 67.3% yield) as a yellow solid. LCMS (ESI): [M+H]+=260.1.
References:
US2018/282328,2018,A1 Location in patent:Paragraph 1592; 1593
2199-44-2
148 suppliers
$8.00/1g

86614-23-5
7 suppliers
$134.31/250mgs: