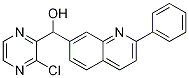
(3-chloropyrazin-2-yl)(2-phenylquinolin-7-yl)Methanol synthesis
- Product Name:(3-chloropyrazin-2-yl)(2-phenylquinolin-7-yl)Methanol
- CAS Number:867162-41-2
- Molecular formula:C20H14ClN3O
- Molecular Weight:347.8
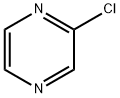
14508-49-7
376 suppliers
$14.00/25g
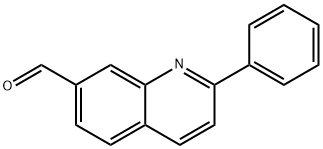
867162-43-4
29 suppliers
$189.00/250mg

867162-41-2
6 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: 2-chloropyrazinwith 2,2,6,6-tetramethyl-piperidine;n-butyllithium in tetrahydrofuran;hexanes at -78;Cooling with acetone-dry ice;Cooling with an ice/water;
Stage #2: 2-phenylquinoline-7-carbaldehyde in tetrahydrofuran;hexanes at -78 - 0; for 2.58333 h;
Stage #3: with citric acid in tetrahydrofuran;hexane;water;
Steps:
To a solution of 2,2,6,6-tetramethylpiperidine (0.82OmL, 0.686g, 4.86mmol) in dry THF (15mL), cooled by Cθ2(s)/acetone, is added nBuLi (2.5M in hexanes; 1.95mL, 4.88mmol). The cooling bath is replaced with an ice/water bath for 15min, and then the solution is re-cooled to -780C. After 5min, a solution of 2-chloropyrazine (VIII) (0.37OmL, 0.475g, 4.14mmol) in THF (0.5mL) is added. 25min later, a solution of 2-phenylquinoline-7- carbaldehyde (compound of Formula Q^-CHO where Q1 = 2-phenylquinolin-7-yl) (890mg, 3.82mmol) in dry THF (7mL) is added slowly over 5min from a syringe which is then rinsed with THF (ImL), and the mixture is stirred at -780C for 2h and then warmed up to O0C for 0.5h. The reaction is quenched by adding citric acid (0.25M aqueous solution). The mixture is extracted with EtOAc (4x30mL), and the combined EtOAc extracts are washed with water, sodium bicarb solution, and brine and dried over MgSO4. The crude material is chromatographed on silica gel [Jones Flashmaster, 5Og / 15OmL cartridge, eluting with CH2Cl2 (4x50mL, then 1-16) → 2% EtOAc in CH2Cl2 (17-30) → 5% (31-59) → 7% (60- 85) → 10% (86-110)] to obtain the title compound as an off-white foam. 1H NMR (CDCl3, 400 MHz): δ = 4.80 (d, J= 7.6 Hz, IH), 6.25 (d, J= 7.6 Hz, IH), 7.43-7.56 (m, 3H), 7.58 (dd, J= 1.8, 8.2 Hz, IH), 7.83 (d, J= 8.4 Hz, IH), 7.87 (d, J= 8.4 Hz, IH), 8.06 (brs, IH), 8.10-8.15 (m, 2H), 8.20 (d, J= 8.4 Hz, IH), 8.41 (d, J= 2.4 Hz, IH), 8.62 (d, J= 2.4 Hz, IH). MS (ES+): m/z 348.0/350.0 (100/37) [MH+]. HPLC: fa = 3.3 min (MicromassZQ, polar_5min).
References:
WO2009/91939,2009,A1 Location in patent:Page/Page column 60

867162-43-4
29 suppliers
$189.00/250mg

867162-41-2
6 suppliers
inquiry