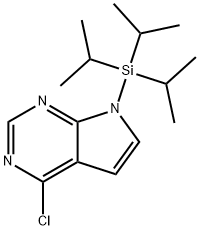
4-chloro-7-[tris(1-methylethyl)silyl]-7H-Pyrrolo[2,3-d]pyrimidine synthesis
- Product Name:4-chloro-7-[tris(1-methylethyl)silyl]-7H-Pyrrolo[2,3-d]pyrimidine
- CAS Number:870706-50-6
- Molecular formula:C15H24ClN3Si
- Molecular Weight:309.91
![4-Chloro-7H-pyrrolo[2,3-d]pyrimidine](/CAS/GIF/3680-69-1.gif)
3680-69-1
808 suppliers
$6.00/1g
![4-chloro-7-[tris(1-methylethyl)silyl]-7H-Pyrrolo[2,3-d]pyrimidine](/CAS/20180703/GIF/870706-50-6.gif)
870706-50-6
16 suppliers
inquiry
Yield:870706-50-6 100%
Reaction Conditions:
Stage #1: 4-chloro-1H-pyrrolo[2,3-d]pyrimidinewith n-butyllithium in tetrahydrofuran;hexane at -78 - 20; for 5.5 h;
Stage #2: with water;ammonium chloride in tetrahydrofuran;hexane;
Steps:
40.40A
Example 40; 40A. 4-Chloro-7-triisoDroDYlsilanyl-7H-Dyrrolo12,3-dlDyrimidine nBuLi (143 mL, 2.5 M in Hexane, 358 mmol) was added slowly to a solution of 4- Chloro-7H-pyrrolo[2,3-d]pyrimidine (50 g, 326 mmol) in THF (1.0 L) at -78 °C and stirred for 1 h. 3-tert-Butyl-3-chloro-2,2,4,4-tetramethyl-pentane (69.1 g, 358 mmol) was slowly added and the reaction mixture, stirred for 1.5 h at -78 °C , warmed to room temperature, and stirred for 3 h. The reaction mixture was quenched with 5% NH4CI (300 mL), concentrated in vacuo to remove THF, and extracted with CH2CI2 (4x500 mL). The combined organic extracts were filtered through silica gel and concentrated in vacuo to afford the title compound as a brown oil (100 g, 100%). MS: 310.1 (MH+); HPLC Rf: 9.40 min. (HPLC method 4).
References:
WO2005/116035,2005,A1 Location in patent:Page/Page column 63
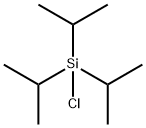
13154-24-0
425 suppliers
$20.00/10g
![4-Chloro-7H-pyrrolo[2,3-d]pyrimidine](/CAS/GIF/3680-69-1.gif)
3680-69-1
808 suppliers
$6.00/1g
![4-chloro-7-[tris(1-methylethyl)silyl]-7H-Pyrrolo[2,3-d]pyrimidine](/CAS/20180703/GIF/870706-50-6.gif)
870706-50-6
16 suppliers
inquiry