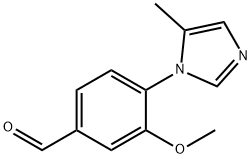
Benzaldehyde, 3-methoxy-4-(5-methyl-1H-imidazol-1-yl)- synthesis
- Product Name:Benzaldehyde, 3-methoxy-4-(5-methyl-1H-imidazol-1-yl)-
- CAS Number:870837-19-7
- Molecular formula:C12H12N2O2
- Molecular Weight:216.24
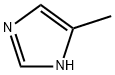
822-36-6
477 suppliers
$9.00/1g
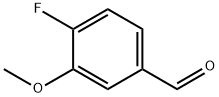
128495-46-5
222 suppliers
$8.00/1g
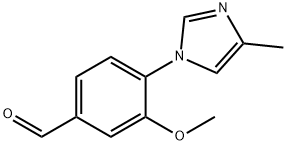
870837-18-6
71 suppliers
$64.00/250mg

870837-19-7
0 suppliers
inquiry
Yield:-
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 100;Product distribution / selectivity;
Steps:
2
Reference Example 2 Synthesis of 3-methoxy-4-(4-methyl-1H-imidazol-1-yl)benzaldehyde Synthesis of 3-methoxy-4-(4-methyl-1H-imidazol-1-yl)benzaldehyde and 3-methoxy-4-(5-methyl-1H-imidazol-1-yl)benzaldehyde Potassium carbonate (4.05 g) was added to a solution of 4-fluoro-3-methoxybenzaldehyde (3.00 g) and 4-methylimidazole (3.307 g) in DMF (50 mL) and the reaction solution was stirred at 100°C overnight. The resulting reaction mixture was concentrated under reduced pressure. Water and ethyl acetate were added to the residue and the organic layer was separated. The organic layer was washed with brine and then dried over anhydrous magnesium sulfate and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (elution solvent: hexane-ethyl acetate system) to provide 3-methoxy-4-(4-methyl-1H-imidazol-1-yl)benzaldehyde (856 mg) and 3-methoxy-4-(5-methyl-1H-imidazol-1-yl)benzaldehyde (44 mg). The property values of 3-methoxy-4-(4-methyl-1H-imidazol-1-yl)benzaldehyde are as follows. 1H-NMR (CDCl3) δ(ppm): 2.31 (s, 3H), 3.97 (s, 3H), 7.02 (brs, 1H), 7.44 (d, J = 8.0 Hz, 1H), 7.55 (dd, J = 1.6 Hz, 8.0 Hz, 1H), 7.58 (d, J = 1.6 Hz, 1H), 7.84 (brs, 1H), 10.00 (s, 1H), The property values of 3-methoxy-4-(5-methyl-1H-imidazol-1-yl)benzaldehyde are as follows. 1H-NMR (CDCl3 ) δ(ppm) : 2.10 (s, 3H), 3.90 (s, 3H), 6.91 (brs, 1H), 7.40 (d, J = 8.0 Hz, 1H), 7.50 (d, J = 1.2 Hz, 1H), 7.57-7.59 (m, 1H), 7.84 (s, 1H), 10.05 (s, 1H), 3-Methoxy-4-(4-methyl-1H-imidazol-1-yl)benzaldehyde can also be separately synthesized by the following method.
References:
EP1953151,2008,A1 Location in patent:Page/Page column 14