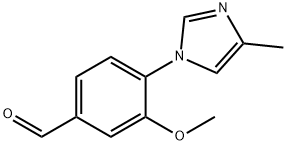
3-Methoxy-4-(4-methyl-1H-imidazol-1-yl)benzaldehyde synthesis
- Product Name:3-Methoxy-4-(4-methyl-1H-imidazol-1-yl)benzaldehyde
- CAS Number:870837-18-6
- Molecular formula:C12H12N2O2
- Molecular Weight:216.24
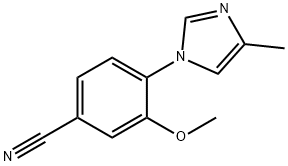
1243204-92-3
27 suppliers
inquiry

870837-18-6
70 suppliers
$64.00/250mg
Yield:870837-18-6 64.6%
Reaction Conditions:
Stage #1: 3-methoxy-4-(4-methyl-1H-imidazol-1-yl)benzonitrilewith bis(2-methoxyethoxy)aluminum hydride in tetrahydrofuran;toluene at -10;Inert atmosphere;
Stage #2: with hydrogenchloride;water in tetrahydrofuran;toluene at 7 - 20;
Steps:
5
3-Methoxy-4-(4-methyl-lH-imidazol-l-yl)benzonitrile (3.50 g, 16.4 mmol) was suspended in tetrahydrofuran (31.6 ml) in a nitrogen gas atmosphere at -10°C and then, to the resultant suspension were added dropwise a Vitride (TM, manufactured by Sigma- Aldrich Corporation) toluene solution (65 wt%, 3.47 g, 11.2 mmol) using a syringe. After completion of the dropwise addition, the solution was stirred at -10°C for one hour, and disappearance of the raw material was confirmed by TLC (silica gel; developing solvent: ethyl acetate; UV detector). Acetone (0.26 mL, 3.58 mmol) was added to the reaction mixture and stirred for 10 minutes, and then the resultant mixture was added dropwise to 5 M hydrochloric acid (18 mL) cooled at 7°C while stirring, followed by temperature elevation to room temperature. The resultant solution was added dropwise to a mixture of a 5 M aqueous sodium hydroxide solution (20.4 mL) and toluene (32 mL), which was cooled to 7°C in advance, while stirring, and the temperature of the resultant mixture was elevated to room temperature. The lower layer (aqueous layer) was separated from the organic layer, the aqueous layer was further extracted with toluene (18 mL) and the toluene layer and the above organic layer were combined. The combined organic layer was washed with a 10% aqueous sodium chloride solution (18 mL x 4) and then filtered through a Celite (1 g) pad, and the resultant filtrate was concentrated under a reduced pressure at a water bath temperature of 50°C. The residue was further subjected to azeotropic distillation with toluene under a reduced pressure to obtain 3.70 g of a crude product containing a title compound.The obtained crude product was dissolved in a mixture of toluene (3.5 mL) and acetone (7 mL) at 60°C, and n-heptane (15.8 mL) was slowly added dropwise to the resultant solution while stirring so that the internal temperature was maintained at 50°C or higher. After completion of the dropwise addition, the solution was subjected to crystallization (seed crystal: lotNo. A6103102) at 53°C, the water bath was removed and the slurry was gradually cooled to room temperature and stirred overnight. The resultant slurry was further cooled to 7°C and stirred for 9 hours and 30 minutes, and then the solids were collected by filtration and washed with an acetone/n-heptane (1/3) mixture which was cooled to 7°C in advance, and dried under a reduced pressure at 45 to 50°C for 1.5 hours to obtain 2.64 g of a title compound (yield: 74.4%). The quantitative determination by HPLC showed that the loss of the compound into the mother liquor was 0.435 g (12.3%).The above-obtained title compound (2.64 g) and the mother liquor (containing 0.435 g of the compound) were mixed together, the mixture was concentrated, the concentrate was dissolved in a mixture of toluene (3 mL), acetone (6 mL), and tert-butyl methyl ether (18 mL), which was warmed at 48°C, and then the resultant solution was cooled to 42°C to perform a crystallization. The resultant slurry was gradually cooled to room temperature and stirred overnight. To the slurry was further added dropwise tert-butyl methyl ether (9 mL), and then the resultant mixture was stirred for one hour, cooled to 8°C and stirred for one hour, followed by stirring at 4°C overnight (17 hours). The solids were collected by filtration and dried under a reduced pressure to obtain 1.97 g of a title compound (yield: 64.6%). The quantitative determination by HPLC showed that the loss of the compound into the mother liquor was 0.674 g (22%).
References:
WO2011/37244,2011,A1 Location in patent:Page/Page column 8; 13; 14
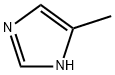
822-36-6
476 suppliers
$9.00/1g
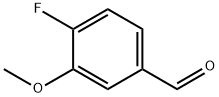
128495-46-5
220 suppliers
$8.00/1g

870837-18-6
70 suppliers
$64.00/250mg
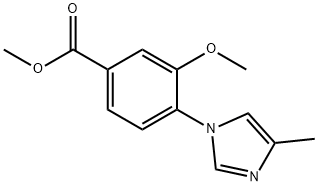
870837-21-1
28 suppliers
$328.00/250mg

870837-18-6
70 suppliers
$64.00/250mg

822-36-6
476 suppliers
$9.00/1g

128495-46-5
220 suppliers
$8.00/1g

870837-18-6
70 suppliers
$64.00/250mg
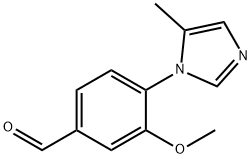
870837-19-7
0 suppliers
inquiry
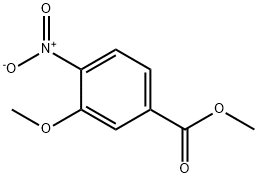
5081-37-8
118 suppliers
$50.10/1g

870837-18-6
70 suppliers
$64.00/250mg