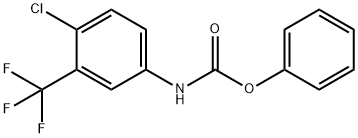
Carbamic acid,N-[4-chloro-3-(teifluoromethyl)phenyl]-,phenyl ester synthesis
- Product Name:Carbamic acid,N-[4-chloro-3-(teifluoromethyl)phenyl]-,phenyl ester
- CAS Number:871555-75-8
- Molecular formula:C14H9ClF3NO2
- Molecular Weight:315.67
Yield:871555-75-8 95%
Reaction Conditions:
with triethylamine in dichloromethane at 5 - 20; for 2 h;
Steps:
1 Example 1: Synthesis of phenyl N-(4-chloro-3-(trifluoromethyl)phenyl)carbamate (3)
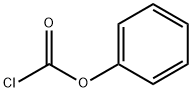
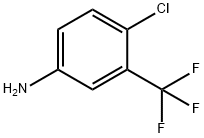
Add 4-chloro-3-(trifluoromethyl)aniline (2) (195.5 g) and triethylamine (202.4g) to dichloromethane (1000 mL), cool the system to 5, and dropwise add phenyl chloroformate(187.8g), after dripping, warm to room temperature and stir for 2h. After the reaction, the system is diluted with dichloromethane (1500ml), water (2000ml) is added, and the liquids are separated. The organic phase is sequentially water (1000mL) and saturated chlorine Wash with sodium chloride solution (1000ml), dry with anhydrous sodium sulfate, concentrate under reduced pressure, and recrystallize (ethyl acetate/n-hexane=1/10 weight ratio) to obtain 286.5 g of compound 3 with a yield of 95%.This step uses the dichloromethane/triethylamine system to replace the N,N-dimethylformamide/pyridine system, avoids the use of more toxic pyridine, simplifies the post-treatment method, does not use the hydrochloric acid aqueous solution for treatment, reduces the cost and reduces Discharge of waste liquid.
References:
CN111718294,2020,A Location in patent:Paragraph 0016-0017