
2,6-DIMETHYL-4'-(TRIFLUOROMETHYL)-1,1'-BIPHENYL-4-OL synthesis
- Product Name:2,6-DIMETHYL-4'-(TRIFLUOROMETHYL)-1,1'-BIPHENYL-4-OL
- CAS Number:872258-58-7
- Molecular formula:C15H13F3O
- Molecular Weight:266.26

7463-51-6
257 suppliers
$9.00/10g
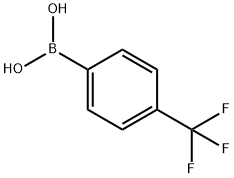
128796-39-4
422 suppliers
$10.00/5g

872258-58-7
15 suppliers
$214.24/250mg
Yield:872258-58-7 87.3%
Reaction Conditions:
with potassium fluoride;palladium diacetate;bis[2-(diphenylphosphino)phenyl] ether in tetrahydrofuran; for 18 h;Heating / reflux;Suzuki Coupling;
Steps:
1
Preparation 12,6-dimethyl-4'-(trifluoromethyl)biphenyl-4-ol4-Bromo-3,5-dimethylphenol (115.00 g, 571.96 mmol), 4-(trifluoromethyl)phenyl boronic acid (130.36 g, 686.35 mmol), (oxydi-2,l-phenylene)bis(diphenylphosphine) (126.00 g, 233.96 mmol), potassium fluoride (99.69 g, 1.72 mol), and Pd(OAc)2 (25.68 g, 114.39 mmol) are added to nitrogen-sparged tetrahydrofuran (3.0 L) and heated to reflux. The consumption of the starting material, 4-bromo-3,5-dimethylphenol, is monitored by GC. Reflux is maintained until 4-bromo-3,5-dimethylphenol has been consumed and is generally complete after 18 h. After the reaction is complete, the batch is cooled to approximately 25 0C. The crude reaction mixture is absorbed onto silica (-500 g) and eluted over silica (1.5 kg) with 10% ethyl acetate in heptane to obtain the product as a solid (132.9 g, 87.3%). The product is crystallized from heptane (23 L/kg) and isopropanol (0.4 L/kg) to yield the title compound (119.5 g; 78.5% yield) as an off-white
References:
WO2007/120284,2007,A2 Location in patent:Page/Page column 29-30
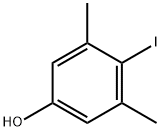
80826-86-4
97 suppliers
$16.00/250mg

128796-39-4
422 suppliers
$10.00/5g

872258-58-7
15 suppliers
$214.24/250mg