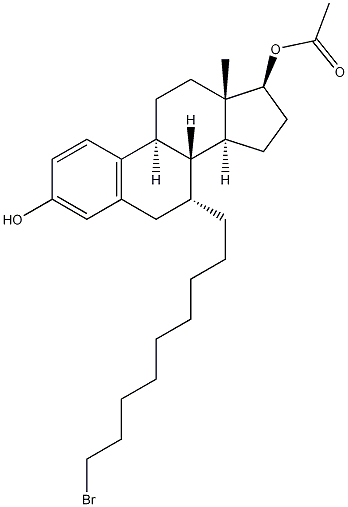
(7a,17b)-7-(9-Bromononyl)-estra-1,3,5(10)-triene-3,17-diol 17-acetate synthesis
- Product Name:(7a,17b)-7-(9-Bromononyl)-estra-1,3,5(10)-triene-3,17-diol 17-acetate
- CAS Number:875573-66-3
- Molecular formula:C29H43BrO3
- Molecular Weight:519.55
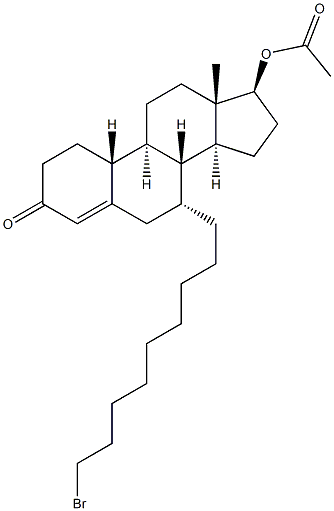
875573-63-0
30 suppliers
$1066.00/1g

875573-66-3
143 suppliers
$117.00/5mg
Yield:-
Reaction Conditions:
with lithium bromide;copper(ll) bromide in acetonitrile at 20; for 7 h;
Steps:
9
Example 9; Preparation of Cp 9342 by Aromatization of Cp 9341 (Depicted in FIG. 6; 37 grams of Cp 9341 and 6.15 grams of lithium bromide are dissolved in 629 grams of acetonitrile and 18.5 grams of copper (II) bromide added. The mixture was stirred at ambient temperature for 7 hours, and then diluted with a solution of 74 grams of ammonium chloride in 942 grams of water. The mixture is further diluted with 37 grams of ammonium hydroxide solution 25% and 185 grams of ethyl acetate and left overnight under agitation in an open flask. The phases are separated and the upper (organic) phase is evaporated to an oily residue, which is dissolved in 500 grams of dichloromethane and washed with 370 grams of water. The phases are separated and the lower (organic) phase is evaporated to an oily residue. Weight of Cp 9342 obtained: 36.5 g
References:
US2006/30552,2006,A1 Location in patent:Page/Page column 25
89359-54-6
122 suppliers
$29.60/1g

875573-66-3
143 suppliers
$117.00/5mg
2590-41-2
174 suppliers
$50.00/1g

875573-66-3
143 suppliers
$117.00/5mg

55362-80-6
276 suppliers
$10.00/1g

875573-66-3
143 suppliers
$117.00/5mg
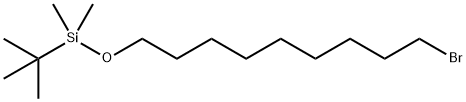
149051-24-1
19 suppliers
inquiry

875573-66-3
143 suppliers
$117.00/5mg