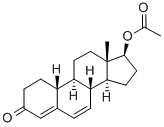
Dehydronandrolone Acetate synthesis
- Product Name:Dehydronandrolone Acetate
- CAS Number:2590-41-2
- Molecular formula:C20H26O3
- Molecular Weight:314.42

4999-76-2
8 suppliers
inquiry

2590-41-2
179 suppliers
$50.00/1g
Yield:2590-41-2 79%
Reaction Conditions:
Stage #1: 3,17β-diacetoxyoestra-3,5-dienewith N-Bromosuccinimide in DMF (N,N-dimethyl-formamide);water at -10 - 25; for 2.5 h;
Stage #2: with lithium carbonate;lithium bromide in DMF (N,N-dimethyl-formamide);water at 80 - 85; for 3 h;
Steps:
2 Example 2
To a suspension of compound (3A) (0.200 kg) in DMF (0.755 kg) and water (0. 0124 kg) AT-10 C to-5°C was added a solution of N-BROMOSUCCINIMIDE (0. 107kg) in DIMETHYLFORMAMIDE (DMF) (0. 330 KG) drop wise over 2 hours whilst MAINTAINING THE TEMPERATURE BELOW 0 C. The reaction mixture was allowed to warm to 20 to 25 C over a 30 minute period and monitored by HPLC. Upon completion of the reaction, lithium carbonate (0.099 kg) and lithium bromide (0.051 kg) were added sequentially with thorough stirring. The reaction MIXTURE WAS SIOWLY HEATED TO 80 C OVER 1 HOUR AND MAINTAINED at 805 C for 2 to 3 hours until the reaction was complete. Heating was then stopped and the BEIGE/BROWN suspension was cooled to 20 to 25 C. The mixture was quenched by the drop wise addition of aqueous acetic acid (0. 177KG in 1.11 kg water). Shortly after the addition commenced, the mixture was seeded with compound (5A) (0.001 kg). Finally, the remaining aqueous acetic acid was added and the mixture was stirred at room temperature overnight. The solid was isolated by filtration and the filter cake was washed initially with a 1: 1 mixture of DMF and purified water (0.142 kg DMF in 0. 150 kg water), and finally with purified water (3 x 0.200 kg). The crude solid was suspended in isopropanol (0.365 kg) and heated to 45 C to form a brown solution. Purified water was added drop wise over a period of at least 30 minutes to precipitate the product. The slurry was cooled to 0 to 5 C over 1 hour and was stirred at this temperature for 1 hour. The product-was isolated by filtration and filter cake was washed with a cold (0 to 5 C) mixture of isopropanol (0.04 kg) and purified water (0.060 kg) to give a pale yellow coloured powder. The purified solid was dried to constant weight under vacuum at 40 to 50 C (yield : 79%).
References:
WO2004/78774,2004,A1 Location in patent:Page 33-34
14531-84-1
26 suppliers
$788.00/200mg

108-24-7
0 suppliers
$14.00/250ML

2590-41-2
179 suppliers
$50.00/1g

434-22-0
257 suppliers
$33.00/1mg

2590-41-2
179 suppliers
$50.00/1g
1425-10-1
21 suppliers
inquiry

2590-41-2
179 suppliers
$50.00/1g
1164-99-4
2 suppliers
inquiry

2590-41-2
179 suppliers
$50.00/1g