
Fulvestrant synthesis
- Product Name:Fulvestrant
- CAS Number:129453-61-8
- Molecular formula:C32H47F5O3S
- Molecular Weight:606.77
![(7a,17b)-7-[9-[(4,4,5,5,5-Pentafluoropentyl)thio]nonyl]-estra-1,3,5(10)-triene-3,17-diol](/CAS2/GIF/153004-31-0.gif)
153004-31-0
109 suppliers
$498.06/5MG

129453-61-8
470 suppliers
$18.00/1mg
Yield:129453-61-8 87%
Reaction Conditions:
Stage #1:(+)-(7α)-[9-(4,4,5,5,5-pentafluoropentylsulfanyl)nonyl]estra-1,3,5(10)-triene-3,17β-diol with β‐cyclodextrin in water;acetone at 20 - 60; for 0.5 h;
Stage #2: with N-Bromosuccinimide in water;acetone at 40; for 10 h;Temperature;
Steps:
2 2. Preparation of fulvestrant
0.5 g (0.44mmol) β- cyclodextrin was added to 100mL water,The system was heated to 60 ° C and stirred.The compound of formula I (1.3 g, 2.2 mol) was then dissolved in 30 mL of acetone,Β- cyclodextrin under stirring to an aqueous solution of the acetone solution was added slowly the compound of formula I.After the addition, the system was cooled to room temperature and stirred for 30 minutes.Subsequently, 0.39 g of N-bromosuccinimide (2.2 mmol) was added to the reaction system,After the addition, the temperature of the system was raised to 40 ° C and the mixture was stirred until the HPLC showed that the compound of the formula I was completely reacted (complete reaction in 10 hours) (HPLC showed no peroxide generation).Subsequently, the reaction mixture was carefully concentrated on a rotary evaporator to remove the acetone solvent,The residue was added with 250 mL of ethyl acetate,The filtrate was cooled to about 0 ° C,The β-cyclodextrin was again removed by filtration.The organic phase was separated,The organic phase was dried over anhydrous sodium sulfate and concentrated under reduced pressure.The residue was purified by column chromatography to give fulvestrant (1.16 g, 87%, HPLC purity 99.8%) by recrystallization of the crude product obtained in a mixed solvent of ethyl acetate / methyl t-butyl ether.
References:
Wisdom Pharmaceutical Co., Ltd.;Qiu, Xiaolong;Zhang, Xingang;Wang, Donghui;Zou, Ping;You, Zhengwei;Deng, Xianming;Wang, Junqiang;Jiang, Zhongxing;Hu, Lin;Cao, Lei;Liu, Shikui;Zhang, Yisen CN106279342, 2017, A Location in patent:Paragraph 0017
![Estra-1,3,5(10)-triene-3,17-diol,7-[9-[(4,4,5,5,5-pentafluoropentyl)thio]nonyl]-,17-acetate,(7a,17b)-](/CAS2/GIF/875573-69-6.gif)
875573-69-6
136 suppliers
inquiry

129453-61-8
470 suppliers
$18.00/1mg
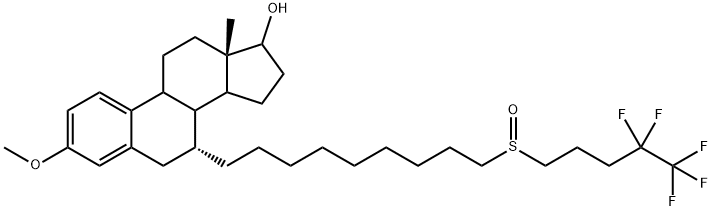
1221256-46-7
13 suppliers
inquiry

129453-61-8
470 suppliers
$18.00/1mg
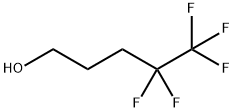
148043-73-6
285 suppliers
$15.00/2 g

129453-61-8
470 suppliers
$18.00/1mg
![(7a,17b)-7-7-[9-[(4,4,5,5,5-Pentafluoropentyl)sulfinyl]nonyl]-estra-1,3,5(10)-triene-3,17-diol 17-acetate](/CAS/20180808/GIF/261506-24-5.gif)
261506-24-5
8 suppliers
inquiry

129453-61-8
470 suppliers
$18.00/1mg