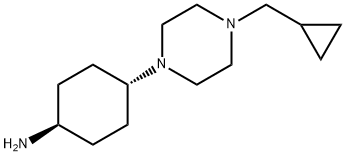
CyclohexanaMine, 4-[4-(cyclopropylMethyl)-1-piperazinyl]-, trans- synthesis
- Product Name:CyclohexanaMine, 4-[4-(cyclopropylMethyl)-1-piperazinyl]-, trans-
- CAS Number:876461-31-3
- Molecular formula:C14H27N3
- Molecular Weight:237.38
![Benzenemethanamine, N-[trans-4-[4-(cyclopropylmethyl)-1-piperazinyl]cyclohexyl]-N-(phenylmethyl)-](/CAS/20210305/GIF/755039-89-5.gif)
755039-89-5
0 suppliers
inquiry
![CyclohexanaMine, 4-[4-(cyclopropylMethyl)-1-piperazinyl]-, trans-](/CAS/GIF/876461-31-3.gif)
876461-31-3
18 suppliers
inquiry
Yield:876461-31-3 100%
Reaction Conditions:
with hydrogen;acetic acid;palladium 10% on activated carbon in methanol at 20 - 30; under 2280.15 Torr; for 12 h;
Steps:
16.2
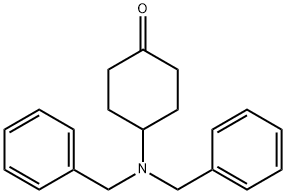
Step 2 (trans)-4-[4-(Cyclopropylmethyl)piperazin-1-yl]cyclohexanamine; (trans)-N,N-Dibenzyl-4-[4-(cyclopropylmethyl)piperazin-1-yl]cyclohexanamine 16a (1.41 g, 3.38 mmol) was dissolved in 15 mL of methanol followed by the addition of palladium / carbon (706 mg, 10%) and 0.1 mL of acetic acid, filled with hydrogen three times. The reaction mixture was reacted for 12 hours at 3 atomosphere of hydrogen, added with 3 g alkaline aluminum oxide (200-300 mesh) and filtered. The filtrate was concentrated under reduced pressure to obtain the title compound (trans)-4-[4-(cyclopropylmethyl)piperazin-1-yl]cyclohexanamine 16b (900 mg, yield: 100%) as a colorless solid. MS m/z (ESI): 238.2 [M+1]
References:
EP2481739,2012,A1 Location in patent:Page/Page column 48
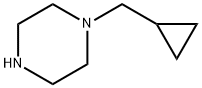
57184-25-5
141 suppliers
$19.00/250mg
149506-79-6
51 suppliers
$16.00/250mg
![CyclohexanaMine, 4-[4-(cyclopropylMethyl)-1-piperazinyl]-, trans-](/CAS/GIF/876461-31-3.gif)
876461-31-3
18 suppliers
inquiry
![CyclohexanaMine, 4-[4-(cyclopropylMethyl)-1-piperazinyl]-, cis-](/CAS/GIF/755039-90-8.gif)
755039-90-8
9 suppliers
inquiry
882660-40-4
9 suppliers
inquiry
![CyclohexanaMine, 4-[4-(cyclopropylMethyl)-1-piperazinyl]-, trans-](/CAS/GIF/876461-31-3.gif)
876461-31-3
18 suppliers
inquiry

149506-79-6
51 suppliers
$16.00/250mg
![CyclohexanaMine, 4-[4-(cyclopropylMethyl)-1-piperazinyl]-, trans-](/CAS/GIF/876461-31-3.gif)
876461-31-3
18 suppliers
inquiry
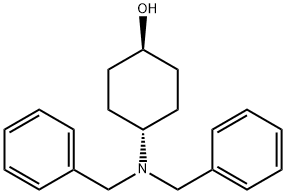
149506-81-0
20 suppliers
inquiry
![CyclohexanaMine, 4-[4-(cyclopropylMethyl)-1-piperazinyl]-, trans-](/CAS/GIF/876461-31-3.gif)
876461-31-3
18 suppliers
inquiry