
CyclohexanaMine, 4-[4-(cyclopropylMethyl)-1-piperazinyl]-, cis- synthesis
- Product Name:CyclohexanaMine, 4-[4-(cyclopropylMethyl)-1-piperazinyl]-, cis-
- CAS Number:755039-90-8
- Molecular formula:C14H27N3
- Molecular Weight:237.38
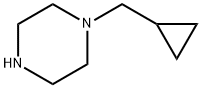
57184-25-5
141 suppliers
$19.00/250mg
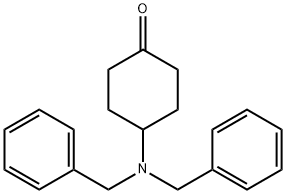
149506-79-6
53 suppliers
$16.00/250mg
![CyclohexanaMine, 4-[4-(cyclopropylMethyl)-1-piperazinyl]-, trans-](/CAS/GIF/876461-31-3.gif)
876461-31-3
18 suppliers
inquiry
![CyclohexanaMine, 4-[4-(cyclopropylMethyl)-1-piperazinyl]-, cis-](/CAS/GIF/755039-90-8.gif)
755039-90-8
9 suppliers
inquiry
Yield:755039-90-8 61% ,876461-31-3 13%
Reaction Conditions:
with sodium tris(acetoxy)borohydride;potassium carbonate in dichloromethane at 20; for 12 h;
Steps:
9.8 g (33.4 mmol) of 4-dibenzylcyclohexanone is dissolved in 100 mL dichloromethane and stirred for 12 h at RT with 5.6 g (40 mmol) of N-cyclopropylmethylpiperazine and 8.5 g (40 mmol) Of NaBH(OAc)3. Then water and potassium carbonate are added, the organic phase is separated off, dried and the solvent is eliminated in vacuo. The residue is purified over a silica gel column (about 50 mL silica gel, about 3 L ethyl acetate 95/ methanol 5 + 0.25% concentrated ammonia. The appropriate fractions are evaporated down in vacuo. The faster eluting cis compound crystallised from ethyl acetate. The trans-compound is crystallised from ethanol + concentrated HCl. Yield: 8.5 g (61%) cis-isomer and 2.2 (13%) trans-isomer.
References:
WO2006/18182,2006,A1 Location in patent:Page/Page column 73 WO2006/18185,2006,A2 Location in patent:Page/Page column 73

149506-79-6
53 suppliers
$16.00/250mg
![CyclohexanaMine, 4-[4-(cyclopropylMethyl)-1-piperazinyl]-, cis-](/CAS/GIF/755039-90-8.gif)
755039-90-8
9 suppliers
inquiry
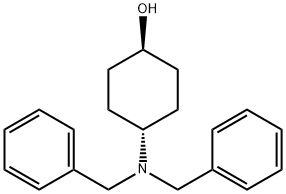
149506-81-0
20 suppliers
inquiry
![CyclohexanaMine, 4-[4-(cyclopropylMethyl)-1-piperazinyl]-, cis-](/CAS/GIF/755039-90-8.gif)
755039-90-8
9 suppliers
inquiry
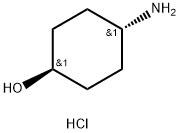
50910-54-8
232 suppliers
$5.00/5g
![CyclohexanaMine, 4-[4-(cyclopropylMethyl)-1-piperazinyl]-, cis-](/CAS/GIF/755039-90-8.gif)
755039-90-8
9 suppliers
inquiry
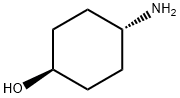
27489-62-9
460 suppliers
$5.00/5g
![CyclohexanaMine, 4-[4-(cyclopropylMethyl)-1-piperazinyl]-, cis-](/CAS/GIF/755039-90-8.gif)
755039-90-8
9 suppliers
inquiry