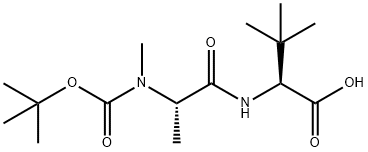
L-Valine, N-[(1,1-dimethylethoxy)carbonyl]-N-methyl-L-alanyl-3-methyl- synthesis
- Product Name:L-Valine, N-[(1,1-dimethylethoxy)carbonyl]-N-methyl-L-alanyl-3-methyl-
- CAS Number:876622-62-7
- Molecular formula:C15H28N2O5
- Molecular Weight:316.39
![L-Valine, N-[(1,1-dimethylethoxy)carbonyl]-N-methyl-L-alanyl-3-methyl-, methyl ester (9CI)](/CAS/20210305/GIF/876623-92-6.gif)
876623-92-6
0 suppliers
inquiry
![L-Valine, N-[(1,1-dimethylethoxy)carbonyl]-N-methyl-L-alanyl-3-methyl-](/CAS/20210111/GIF/876622-62-7.gif)
876622-62-7
1 suppliers
inquiry
Yield:876622-62-7 4600 g
Reaction Conditions:
with lithium hydroxide in tetrahydrofuran;methanol;water at 20; for 18 h;
Steps:
(S)-2-((S)-2-(tert-Butoxycarbonyl(methyl)amino)propanamido)-3,3-dimethylbutanoic acid
To a solution of ((S)-methyl 2-((S)-2-(tert-butoxycarbonyl(methyl)amino)propanamido)-3,3-dimethylbutanoate (5000 g, crude) in THF (50 L) and MeOH (25 L) was added a solution of LiOH (2545 g, 60.6 mol) in H2O (25 L) at room temperature. The mixture was stirred at room temperature for 18 h and then treated with 1M HCl (50 L) and partitioned with EtOAc (25 L). The organic layer was washed with brine, dried over Na2SO4 and concentrated in vacuo to give the title product (4600 g, 99% yield over two steps) as a crystalline solid which was used for the next step without further purification. 1H NMR (CDCl3, 400 MHz): δ = 6.96 - 6.95 (m, 1H), 5.30 - 5.29 (m, 1H), 4.42 (d, J = 9.2 Hz, 1H), 2.79 (s, 3H), 1.48 (s, 9H), 1.32 (d, J = 6.8 Hz, 3H), 0.99 (s, 9H).
References:
Ahmed, Adil;Anderson, Niall A.;Benowitz, Andrew B.;Cryan, Jenni;Dai, Han;McGonagle, Grant A.;Rozier, Christine [Bioorganic and medicinal chemistry letters,2020] Location in patent:supporting information

16948-16-6
312 suppliers
$6.00/1g
![L-Valine, N-[(1,1-dimethylethoxy)carbonyl]-N-methyl-L-alanyl-3-methyl-](/CAS/20210111/GIF/876622-62-7.gif)
876622-62-7
1 suppliers
inquiry
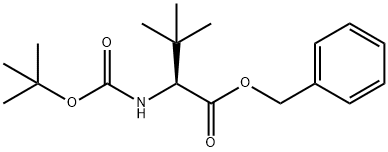
154092-61-2
2 suppliers
inquiry
![L-Valine, N-[(1,1-dimethylethoxy)carbonyl]-N-methyl-L-alanyl-3-methyl-](/CAS/20210111/GIF/876622-62-7.gif)
876622-62-7
1 suppliers
inquiry
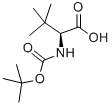
62965-35-9
446 suppliers
$6.00/5g
![L-Valine, N-[(1,1-dimethylethoxy)carbonyl]-N-methyl-L-alanyl-3-methyl-](/CAS/20210111/GIF/876622-62-7.gif)
876622-62-7
1 suppliers
inquiry
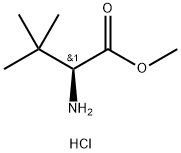
63038-27-7
343 suppliers
$8.00/5g
![L-Valine, N-[(1,1-dimethylethoxy)carbonyl]-N-methyl-L-alanyl-3-methyl-](/CAS/20210111/GIF/876622-62-7.gif)
876622-62-7
1 suppliers
inquiry