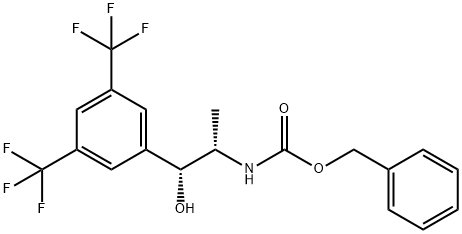
benzyl (1R,2S)-1-(3,5-bis(trifluoromethyl)phenyl)-1-hydroxypropan-2-ylcarbamate synthesis
- Product Name:benzyl (1R,2S)-1-(3,5-bis(trifluoromethyl)phenyl)-1-hydroxypropan-2-ylcarbamate
- CAS Number:877384-16-2
- Molecular formula:C19H17F6NO3
- Molecular Weight:421.33
![CarbaMic acid, N-[(1S)-2-[3,5-bis(trifluoroMethyl)phenyl]-1-Methyl-2-oxoethyl]-, phenylMethyl ester](/CAS/20150408/GIF/871917-79-2.gif)
871917-79-2
4 suppliers
inquiry

877384-16-2
19 suppliers
$288.00/250mg
Yield:877384-16-2 99%
Reaction Conditions:
with aluminum isopropoxide in isopropyl alcohol;toluene at 50; for 15 h;
Steps:
1.2.2-3 [Step 2-3] Preparation of (1 ?,2S)-benzyl-[3,5-bis(trifluoromethyl)phenyl]-1- hydroxypropan-2-yl carbamate
A solution of (S)-benzyl-1-[3,5-bis(trifluoromethyl)phenyl]-1-oxopropan-2-yl carbamate (0.3 g, 0.72 mmol), obtained in step 2-2, in toluene (5.4mL) and isopropyl alcohol (3.6ml_) was added with drops of AI(OPr/)3 (0.22 g, 1.08 mmol) at room temperature, and refluxed at 50 °C for 15 hrs with stirring. The reaction mixture was cooled to room temperature, and the reaction was terminated with 2 N HCI, followed by extraction with ethyl acetate. The organic layer thus formed was dried over anhydrous magnesium sulfate, filtered, and concentrated. The residue was re-crystallized in hexane, and filtered at a reduced pressure to afford the title compound (0.3 g, 99%). H NMR (400 MHz, CDCI3) δ 7.75-7.82 (m, 3H), 7.26-7.40 (m, 5H), 5.12 (s, 2H), 5.03 (brs, 1 H), 4.85 (d, J = 7.2 Hz, 1 H), 4.04 (m, 1 H), 3.24 (brs, H), 0.99 (d, J = 7.2 Hz, 3H).
References:
WO2014/157994,2014,A1 Location in patent:Page/Page column 48
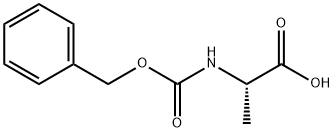
1142-20-7
459 suppliers
$5.00/1g

877384-16-2
19 suppliers
$288.00/250mg

114744-83-1
24 suppliers
inquiry

877384-16-2
19 suppliers
$288.00/250mg

37661-60-2
27 suppliers
$125.00/250mg

877384-16-2
19 suppliers
$288.00/250mg

328-70-1
416 suppliers
$10.00/25g

877384-16-2
19 suppliers
$288.00/250mg