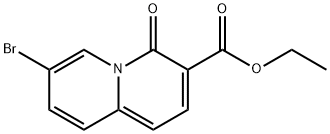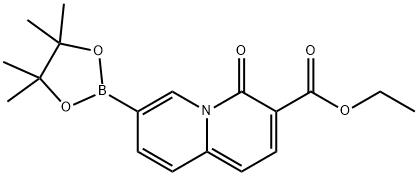
4H-Quinolizine-3-carboxylic acid, 4-oxo-7-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-, ethyl ester synthesis
- Product Name:4H-Quinolizine-3-carboxylic acid, 4-oxo-7-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-, ethyl ester
- CAS Number:880869-92-1
- Molecular formula:C18H22BNO5
- Molecular Weight:343.18
Yield:880869-92-1 70%
Reaction Conditions:
with (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride;potassium acetate in 1,4-dioxane at 80; for 6 h;Inert atmosphere;
Steps:
A.c Step (c Preparation of ethyl 4-oxo-7-(4,4.5,5-tetramethyl-l,3,2-dioxaborolan-2-vD-4H- quinolizine-3-carboxylate (Intermediate Bl)
To a solution of ethyl 7-bromo-4-oxo-quinolizine-3-carboxylate_(2.0l g, 6.79 mmol, compound B1.4) and Pin2B2 (6.95 g, 27.37 mmol) in dioxane (60.0 mL) was added AcOK (2.75 g, 28.02 mmol) and Pd(dppf)Cl2 (148.0 mg, 0.202 mmol), and the resulting mixture was stirred at 80 °C for 6 hr under Argon. After the reaction was cooled back to r.t., the reaction mixture was diluted with water (200 mL) and extracted with DCM (100 mL) three times. The combined organic layer was dried over anhydrous Na2S04, filtered and concentrated in vacuo to give a crude material, which was re-dissolved in DCM (100 mL) and filtered. The filtrate was concentrated under reduced pressure to give a crude product, which was triturated with a mixture of MTBE and petroleum ether (50 mL, 10:1) to give ethyl 4-oxo-7-(4, 4,5, 5-tetramethyl- 1,3,2- dioxaborolan-2-yl)-4H-quinolizine-3-carboxylate (2.35 g, 70% yield) as a yellow solid. MS (ESI): 262.0 ([M-82+H]+). (corresponding boronic acid) H NMR (400 MHz, DMSO-<) d ppm: 9.37 (s, 1 H), 8.30-8.28 (d, 1 H), 7.87-7.81 (m, 2 H), 6.87-6.85 (d, 1 H), 4.27-4.21 (q, 2 H), 1.34 (s,l2 H), 1.30 (t, 3 H).
References:
WO2019/228940,2019,A1 Location in patent:Page/Page column 41-43
87-13-8
432 suppliers
$5.00/5g

880869-92-1
0 suppliers
inquiry
3430-13-5
421 suppliers
$5.00/5g

880869-92-1
0 suppliers
inquiry
1121-78-4
289 suppliers
$6.00/5g

880869-92-1
0 suppliers
inquiry