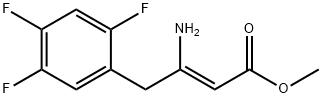
(Z)-Methyl 3-aMino-4-(2,4,5-trifluorophenyl)but-2-enoate synthesis
- Product Name:(Z)-Methyl 3-aMino-4-(2,4,5-trifluorophenyl)but-2-enoate
- CAS Number:881995-70-6
- Molecular formula:C11H10F3NO2
- Molecular Weight:245.2

67-56-1
740 suppliers
$7.29/5ml-f
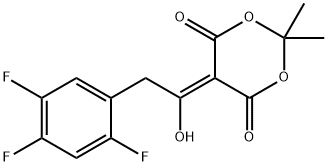
764667-64-3
103 suppliers
$71.00/1g

881995-70-6
34 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1:methanol;5-(1-hydroxy-2-(2,4,5-trifluorophenyl)ethylidene)-2,2-dimethyl-1,3-dioxane-4,6-dione in toluene at 60 - 120; for 72.5 h;
Stage #2: with ammonium acetate in methanol;toluene at 25 - 60; for 24 h;Temperature;
Steps:
1; 3 Example 3
Take 6.00 g of the oily waste produced by the degradation of the key intermediate of sitagliptin and preheat it at 60°C for 30 minutes to obtain the preheated material; dissolve the preheated material in 50 mL of an organic solvent (specifically, methanol-toluene) The mixed solvent, the volume ratio of methanol to toluene is 1:1), heat to reflux under stirring conditions (system temperature is 120°C), maintain reflux state for alcoholysis reaction for 72h (TLC monitor reaction progress); after the reaction, The obtained system was cooled to room temperature (25°C), and then the solvent was recovered by distillation under reduced pressure. The residue was redissolved in 50mL methanol, 8g of ammonium acetate was added, and the mixture was heated to 55-60°C under stirring, and the ammonolysis reaction was carried out for 24h (TLC Monitor the progress of the reaction; after the reaction, the resulting system was cooled to room temperature, and the solvent was recovered by distillation under reduced pressure. The residue was redissolved in 50 mL of ethyl acetate. The resulting mixture was washed 3 times with water (50 mL*3). The organic layer was separated and The solvent was recovered by distillation under reduced pressure, and the residue was purified by column chromatography with a mixture of petroleum ether and ethyl acetate (the volume ratio of petroleum ether and ethyl acetate was 1:1) as the eluent to obtain a white crystal product, which is the recovery Product (76% recovery),The chemical name is (2Z)-3-amino-4-(2,4,5-trifluorophenyl)but-2-enoic acid methyl ester,
References:
CN112341332, 2021, A Location in patent:Paragraph 0050-0051; 0054-0055
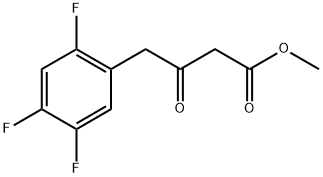
769195-26-8
155 suppliers
$12.00/250mg

881995-70-6
34 suppliers
inquiry
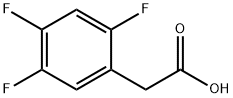
209995-38-0
477 suppliers
$10.00/10 g

881995-70-6
34 suppliers
inquiry

764667-64-3
103 suppliers
$71.00/1g

881995-70-6
34 suppliers
inquiry