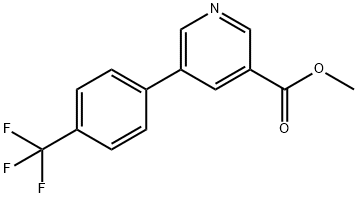
methyl 5-(4-(trifluoromethyl)phenyl)pyridine-3-carboxylate synthesis
- Product Name:methyl 5-(4-(trifluoromethyl)phenyl)pyridine-3-carboxylate
- CAS Number:893734-81-1
- Molecular formula:C14H10F3NO2
- Molecular Weight:281.23

29681-44-5
296 suppliers
$6.00/5g
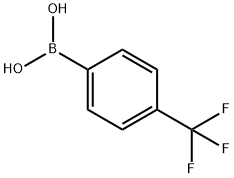
128796-39-4
425 suppliers
$10.00/5g

893734-81-1
10 suppliers
inquiry
Yield:893734-81-1 85%
Reaction Conditions:
with potassium fluoride;tetrakis(triphenylphosphine) palladium(0) in ethanol;water;toluene at 20;Inert atmosphere;Reflux;Suzuki Coupling;
Steps:
1A methyl 5-[4-(trifluoromethyl)phenyl]pyridine-3-carboxylate
General Method 1A: Suzuki CouplingA mixture of the appropriate bromopyridine in toluene (1.8 ml/mmol) is admixed under argon at RT with tetrakis(triphenylphosphine)palladium (0.02 eq.), with a solution of the appropriate arylboronic acid (1.2 eq.) in ethanol (0.5 ml/mmol) and with a solution of potassium fluoride (2.0 eq.) in water (0.2 ml/mmol). The reaction mixture is stirred under reflux for several hours until the conversion is substantially complete. After addition of ethyl acetate and phase separation, the organic phase is washed once with water and once with saturated aqueous sodium chloride solution, dried (magnesium sulphate), filtered and concentrated under reduced pressure. The crude product is purified by flash chromatography (silica gel 60, eluent: dichloromethane/methanol mixtures).; Example 1AMethyl 5-[4-(trifluoromethyl)phenyl]pyridine-3-carboxylate According to General Method 1A, 28 g (132 mmol) of methyl 5-bromonicotinate and 30 g (158 mmol, 1.2 eq.) of 4-trifluoromethylphenylboronic acid were reacted. Yield: 32 g (85% of theory)LC-MS (Method 8B): Rt=2.27 min; MS (ESIpos): m/z=282 [M+H]+.
References:
US2011/21489,2011,A1 Location in patent:Page/Page column 17; 18