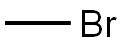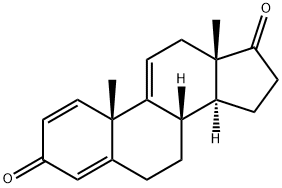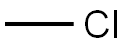
Androsta-1,4,9(11)-triene-3,17-dione, 16-methyl-, (16β)- synthesis
- Product Name:Androsta-1,4,9(11)-triene-3,17-dione, 16-methyl-, (16β)-
- CAS Number:90039-90-0
- Molecular formula:C20H24O2
- Molecular Weight:296.41
Yield:90039-90-0 59.6 g
Reaction Conditions:
with lithium diisopropyl amide in tetrahydrofuran at -70; for 6 h;Inert atmosphere;
Steps:
1
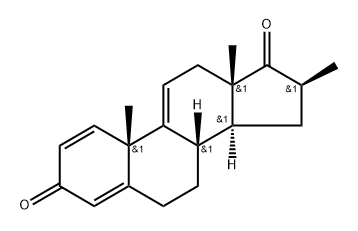
Under nitrogen protection,To a 3000 ml flask was added 300 mL of THF,58.5 g (0.1973 mol) of compound A,Stir and dissolve,Cooling to -70 ,0.9792 mol of lithium diisopropylamine (LDA)A solution of 175.5 g (1.8483 mol) of methyl bromide and 200 mL of THF was added dropwise at -70 ° C,-70 reacted for 6 hours,TLC (PE: EA = 2: 1) showed that the reaction was finished,Dropping glacial acetic acid,Adjust pH = 7 to remove unreacted methyl bromide;To the reaction system was added 450 mL THF,Stirring down to -70 ° C, dropping 0.939 mol of lithium diisopropylamine (LDA)Add after stirring for 10 minutes,14.3 g (0.3635 mol) of trimethylchlorosilane (TMSCl) was added dropwise at -70 ° C,After adding,Insulation -70 reaction 3 hours,TLC (PE: EA = 2: 1) shows that the raw material reaction is complete,Temperature -70 drop by adding 259mL methanol,Add after stirring for 30 minutes,And then at -70 with glacial acetic acid to adjust PH = 7;Naturally rose to room temperature,Add 720mL water to stir and dissolve,The solvent was concentrated under reduced pressure at 50 ° C,Add 180mL petroleum ether,Stirring at room temperature for 2 hours,Filter,After the filter cake was washed with water,And then washed with petroleum ether,dry,Obtained 59.6 g of a pale yellow solid with a mass yield of 102%.
References:
CN106946963,2017,A Location in patent:Paragraph 0040-0041