
4-METHOXY-N-(4H-1,2,4-TRIAZOL-4-YL)BENZENECARBOXAMIDE synthesis
- Product Name:4-METHOXY-N-(4H-1,2,4-TRIAZOL-4-YL)BENZENECARBOXAMIDE
- CAS Number:90916-77-1
- Molecular formula:C10H10N4O2
- Molecular Weight:218.21
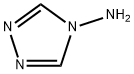
584-13-4
493 suppliers
$5.00/5g
201230-82-2
1 suppliers
inquiry
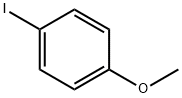
696-62-8
358 suppliers
$5.00/1g

90916-77-1
13 suppliers
inquiry
Yield:90916-77-1 29%
Reaction Conditions:
with palladium diacetate;triethylamine;triphenylphosphine in N,N-dimethyl-formamide at 70; under 750.075 Torr; for 48 h;chemoselective reaction;
Steps:
4.2 Aminocarbonylation of iodoalkenes (1-4) and iodoarenes (5-19) in the presence of nucleophiles a-c under atmospheric carbon monoxide pressure
General procedure: In a typical experiment Pd(OAc)2 (5.6mg, 0.025mmol), triphenylphosphine (13.2mg, 0.05mmol), iodoalkene (1-4) or iodoarene (5-19) (1mmol) were dissolved in DMF (10mL) under argon in a three-necked flask equipped with a reflux condenser and a balloon on the top. Aminotriazole nucleophile (a, b or c) (1.2mmol) and triethylamine (0.5mL) were added. The atmosphere was changed to carbon monoxide. (Caution: High pressure carbon monoxide should only be used with adequate ventilation (hood) using CO sensors as well.) The reaction was conducted for the given reaction time upon stirring at 70°C. The mixture was then concentrated and evaporated to dryness. Toluene (15mL) was added to the residue, the precipitate (product) was filtered, washed with water on the filter and dried. The off-white powder-like material was dissolved in methanol, the palladium-black was filtered off and methanol was evaporated.
References:
Gergely, Máté;Boros, Borbála;Kollár, László [Tetrahedron,2017,vol. 73,# 48,p. 6736 - 6741]