1’-t-Butoxycarbonyl-5-hydroxy-spiro[chroman-2,4’-piperidin]-4-one synthesis
- Product Name:1’-t-Butoxycarbonyl-5-hydroxy-spiro[chroman-2,4’-piperidin]-4-one
- CAS Number:911227-79-7
- Molecular formula:C18H23NO5
- Molecular Weight:333.38
Yield:911227-79-7 74%
Reaction Conditions:
with pyrrolidine in methanol;Heating / reflux;
Steps:
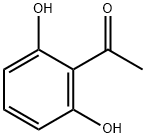
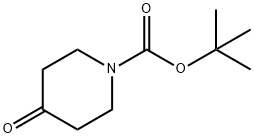
11A.11.1
Preparation of 11.2: 2',6'-dihydroxyacetophenone (11.1) (200.Og g, 1.31 mol, 1.0 eq) was added portion wise at room temperature to pyrrolidine (220 mL, 2.0 eq) followed by portion wise addition of l-Boc-4-piperidone (1.2) (262.0 g, 1.31 mo, 1.0 eq). Anhydrous methanol (100 mL) was then added and the red slurry heated to reflux to dissolve all solids. On dissolution the reaction was cooled to room temperature overnight with stirring to form a solid mass. This solid mass was dissolved in ethyl acetate, washed with a IN aqueous solution of hydrochloric acid, a IN aqueous solution of sodium hydroxide and brine, dried over sodium sulfate and filtered. The solvent was evaporated under vacuum. A mixture of hexane and diethyl ether (80:20) (400 mL) was added to the mixture and the resulting precipitate was collected by filtration, washed with hexane and used for the next step without further purification.Yield: 74%.1H NMR (400MHz, CDCl3) 5 11.61 (s, IH), 7.37 (t, IH), 6.49 (d, IH), 6.44 (d, IH), 3.89 (brs, 2H), 3.20 (brm, 2H), 2.73 (s, 2H), 2.02 (d, 2H), 1.64 (m, 2H), 1.46 (s, 9H) Mass Spectral Analysis m/z = 334.0 (M+H)+
References:
WO2006/105442,2006,A2 Location in patent:Page/Page column 152; 351