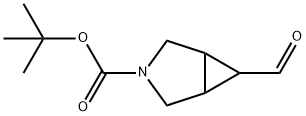
TERT-BUTYL 6-FORMYL-3-AZABICYCLO[3.1.0]HEXANE-3-CARBOXYLATE synthesis
- Product Name:TERT-BUTYL 6-FORMYL-3-AZABICYCLO[3.1.0]HEXANE-3-CARBOXYLATE
- CAS Number:912468-67-8
- Molecular formula:C11H17NO3
- Molecular Weight:211.26
![exo-3-Boc-3-azabicyclo[3.1.0]hexane-6-methanol](/CAS2/GIF/419572-18-2.gif)
419572-18-2
76 suppliers
$52.00/100mg
![TERT-BUTYL 6-FORMYL-3-AZABICYCLO[3.1.0]HEXANE-3-CARBOXYLATE](/CAS/20180629/GIF/912468-67-8.gif)
912468-67-8
4 suppliers
inquiry
Yield:912468-67-8 80%
Reaction Conditions:
with Dess-Martin periodane in dichloromethane at 0; for 1 h;
Steps:
73.3 Step 3
To a solution of (1R,5S,6s)-tert-butyl 6-(hydroxymethyl)-3- azabicyclo[3.1.0]hexane-3-carboxylate (1.20 g, 5.63 mmol, 1.00 eq) in dichloromethane (30.0 mL) was added dess-martin periodinane (2.63 g, 6.19 mmol, 1.92 mL, 1.10 eq) at 0 °C. The mixture was stirred at 0 °C for 1 h. The mixture was diluted with water (5.00 mL) and saturated sodium carbonate (5.00 mL), extracted with dichloromethane (2 × 20.0 mL). The combined organic layers were washed with water (20.0 mL), dried over anhydrous sodium sulfate, filtered. The filtrate was concentrated to afford a residue. The residue was purified by silica gel chromatography (petroleum ether/ethyl acetate = 1/0~4/1) to afford tert-butyl 6-formyl-3-azabicyclo[3.1.0]hexane- 3- carboxylate (0.950 g, 4.50 mmol, 80% yield) as a colorless oil. 1H NMR (400 MHz, Chloroform- d) d = 9.3 (d, J = 6.24 Hz, 1 H), 3.8 - 4.0 (m, 2 H), 3.6 - 3.7 (m, 2 H), 2.1 - 2.2 (m, 2 H), 1.7 - 1.8 (m, 1 H), 1.4 (s, 9 H).
References:
WO2021/30711,2021,A1 Location in patent:Paragraph 1159; 1161
![Tert-Butyl 6-(Hydroxymethyl)-3-Azabicyclo[3.1.0]Hexane-3-Carboxylate](/CAS/20180527/GIF/850808-43-4.gif)
850808-43-4
16 suppliers
$55.00/25mg
![TERT-BUTYL 6-FORMYL-3-AZABICYCLO[3.1.0]HEXANE-3-CARBOXYLATE](/CAS/20180629/GIF/912468-67-8.gif)
912468-67-8
4 suppliers
inquiry

24424-99-5
832 suppliers
$13.50/25G
![TERT-BUTYL 6-FORMYL-3-AZABICYCLO[3.1.0]HEXANE-3-CARBOXYLATE](/CAS/20180629/GIF/912468-67-8.gif)
912468-67-8
4 suppliers
inquiry
![3-AZABICYCLO[3.1.0]HEXAN-6-YLMETHANOL HCL](/CAS/20180529/GIF/850808-44-5.gif)
850808-44-5
3 suppliers
inquiry
![TERT-BUTYL 6-FORMYL-3-AZABICYCLO[3.1.0]HEXANE-3-CARBOXYLATE](/CAS/20180629/GIF/912468-67-8.gif)
912468-67-8
4 suppliers
inquiry
![(1R,5S,6R)-3-(tert-butoxycarbonyl)-3-azabicyclo[3.1.0]hexane-6-carboxylic acid](/CAS/GIF/927679-54-7.gif)
927679-54-7
114 suppliers
$29.00/100mg
![TERT-BUTYL 6-FORMYL-3-AZABICYCLO[3.1.0]HEXANE-3-CARBOXYLATE](/CAS/20180629/GIF/912468-67-8.gif)
912468-67-8
4 suppliers
inquiry