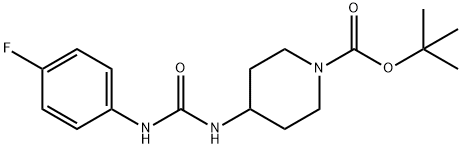
tert-Butyl 4-[3-(4-fluorophenyl)ureido]piperidine-1-carboxylate synthesis
- Product Name:tert-Butyl 4-[3-(4-fluorophenyl)ureido]piperidine-1-carboxylate
- CAS Number:913634-45-4
- Molecular formula:C17H24FN3O3
- Molecular Weight:337.39
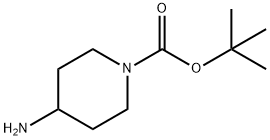
87120-72-7
20 suppliers
$6.00/1g
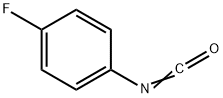
1195-45-5
202 suppliers
$6.00/1g
![tert-Butyl 4-[3-(4-fluorophenyl)ureido]piperidine-1-carboxylate](/CAS/20180601/GIF/913634-45-4.gif)
913634-45-4
8 suppliers
$350.00/1g
Yield:913634-45-4 71%
Reaction Conditions:
in tetrahydrofuran at 20; for 1 - 2 h;
Steps:
II.1
To a solution of compound I-c (5 g, 24.96 mmol) in THF (60 mL) was added compound II-a (R =R =R =R =H, R =F, 4.35 g, 27.43 mmol). The reaction mixture was allowed to stir at room temperature for 1-2 hours. After removing the solvent in vacuo, the residue was purified by column chromatography to give the title compound as a white solid (6 g, 71%).[113] 1H-NMR(CDCl ): δ 7.75-7.81(m, 2H), 7.08-7.27 (m, 2H), 4.09-4.22 (m, 3H),2.85-2.95 (m, 2H), 1.98-2.04 (m, 2H), 1.47 (s, 9H), 1.41-1.45 (m, 2H).
References:
WO2006/115353,2006,A1 Location in patent:Page/Page column 12

79099-07-3
543 suppliers
$5.00/5g
![tert-Butyl 4-[3-(4-fluorophenyl)ureido]piperidine-1-carboxylate](/CAS/20180601/GIF/913634-45-4.gif)
913634-45-4
8 suppliers
$350.00/1g
206273-87-2
80 suppliers
$45.00/50mg
![tert-Butyl 4-[3-(4-fluorophenyl)ureido]piperidine-1-carboxylate](/CAS/20180601/GIF/913634-45-4.gif)
913634-45-4
8 suppliers
$350.00/1g